Abstract
Green tea has been found to increase the lifespan of various experimental animal models including the fruit fly, Drosophila melanogaster. High in polyphenolic content, green tea has been shown to reduce oxidative stress in part by its ability to bind free iron, a micronutrient that is both essential for and toxic to all living organisms. Due to green tea’s iron-binding properties, we questioned whether green tea acts to increase the lifespan of the fruit fly by modulating iron regulators, specifically, mitoferrin, a mitochondrial iron transporter, and transferrin, found in the hemolymph of flies. Publicly available hypomorph mutants for these iron-regulators were utilized to investigate the effect of green tea on lifespan and fertility. We identified that green tea could not increase the lifespan of mitoferrin mutants but did rescue the reduced male fertility phenotype. The effect of green tea on transferrin mutant lifespan and fertility were comparable to w1118 flies, as observed in our previous studies, in which green tea increased male fly lifespan and reduced male fertility. Expression levels in both w1118 flies and mutant flies, supplemented with green tea, showed an up-regulation of mitoferrin but not transferrin. Total body and mitochondrial iron levels were significantly reduced by green tea supplementation in w1118 and mitoferrin mutants but not transferrin mutant flies. Our results demonstrate that green tea may act to increase the lifespan of Drosophila in part by the regulation of mitoferrin and reduction of mitochondrial iron.
Keywords: green tea, iron, lifespan, fertility, mitoferrin
INTRODUCTION
Green tea polyphenols have been found to increase the lifespan of the fruit fly, Drosophila melanogaster (Jimenez-Del-Rio et al., 2010; Li et al., 2007; Lopez et al., 2014; Massie et al., 1993). Despite its well-documented health benefits in humans and among various experimental animal models (i.e., worms, flies and mice), green tea’s life-extending mechanisms are not well understood (Abbas and Wink, 2009; Kitani et al., 2007; Li et al., 2007; Strong et al., 2013). Some reports have suggested that green tea’s iron-binding activity and thus reduction of oxidative stress is the basis of its beneficial health and life-extending effects (Mak, 2012; Massie et al., 1993; Thephinlap et al., 2007; Weinreb et al., 2004). However, the precise relationship between green tea and the modulators of iron metabolism has not been established.
Green tea polyphenols, known as catechins, most notably epigallocatechin gallate (EGCG), have been purported to be responsible for green tea’s numerous biological effects (Sinija and Mishra, 2008; Suzuki et al., 2012; Zaveri, 2006). Phenolic compounds have been described as multi-functional antioxidants with metal-chelating abilities, such as iron binding (Khokhar and Apenten, 2003; Perron and Brumaghim, 2009). Iron is a micronutrient that is both essential for and toxic to all living organisms. Free iron can readily catalyze the generation of reactive oxygen intermediates such as hydroxyl radicals which can lead to cell and tissue damage (Papanikolaou and Pantopoulos, 2005). Iron has also been shown to accumulate during aging and a decrease in aging-induced iron accumulation has been found to increase the lifespan of various experimental animal models (Arruda et al., 2013; Massie et al., 1993; Ventura et al., 2005; Xu et al., 2010). For example, fruit flies supplemented with green tea in their diet throughout their life exhibited longer lifespans and reduced total body iron levels further supporting an interaction between green tea’s iron-binding and lifespan extension properties (Massie et al., 1993).
In addition to chelating iron, green tea may regulate iron homeostasis in flies through the modulation of essential iron regulators, since the process is partially conserved between flies and humans (Tang and Zhou, 2013). Iron homeostasis involves the action of numerous protein regulators. Among them are mitoferrin and transferrin, evolutionary conserved proteins involved in important physiological processes in Drosophila (Mandilaras et al., 2013; Tang and Zhou, 2013). Mitoferrin, located within the inner mitochondrial membrane, is a protein of the mitochondrial solute carrier family known to transport iron into the mitochondria (Mandilaras et al., 2013; Tang and Zhou, 2013). It has previously been reported that a reduction in mitoferrin resulted in abnormal development and increased the lifespan of C. elegans (Ren et al., 2012). In Drosophila, mitoferrin mutants display sterility in males (Metzendorf and Lind, 2010). Further mechanistic studies demonstrated that this sterility could be due to the fact that mitoferrin plays an important role in spermatogenesis and development (Metzendorf and Lind, 2013). Transferrin, found in the plasma of mammals (Gkouvatsos et al., 2012) and hemolymph of fruit flies (Yoshiga et al., 1999), is an endogenous iron-chelator involved in the systemic transport of iron in mammals and plays a protective role in immunity in Drosophila (Mandilaras et al., 2013; Yoshiga et al., 1999). While the function of transferrin in Drosophila may differ from that of mammals, transferrin expression in flies has been shown to be influenced by dietary iron availability (Yoshiga et al., 1999). Thus, green tea catechins, which bind non-transferrin bound iron (NTBI) (Thephinlap et al., 2007) may modulate the expression of transferrin in flies.
Previously, we reported that green tea increased the lifespan of w1118 male Drosophila by 19% while reducing fertility (Lopez et al., 2014). We further identified negative impairments in the reproductive organs of Drosophila treated with green tea, including atrophied testes in male flies (Lopez et al., 2016). In consideration of the inverse relationship between reproduction and lifespan in Drosophila, a well-documented occurrence (Flatt, 2011; Kirkwood and Rose, 1991; Prowse and Partridge, 1997), and the importance of iron for spermatogenesis (Hales, 2010; Metzendorf and Lind, 2010; Tvrda et al., 2015), we questioned whether green tea acts by modulating iron regulation to affect lifespan and fertility. In this study, we investigated the requirement of iron transporters, mitoferrin and transferrin, for lifespan extension and reproductive function by green tea in Drosophila. Using publicly available fruit fly mutants for mitoferrin and transferrin, we evaluated the effect of green tea on various parameters including lifespan, fertility, and iron levels. We observed that mitoferrin and transferrin mutant flies exhibited longer lifespans and reduced fertility compared to a standard laboratory strain, w1118. We found that green tea failed to increase the lifespan of mitoferrin mutants but increased male fertility. We further tested the effect of green tea on a mutant Drosophila strain of transferrin. We found that green tea increased the lifespan and reduced fertility of transferrin mutants, an effect that parallels the action of green tea in w1118 male flies from our previous work (Lopez et al., 2014). In addition, green tea reduced mitochondrial iron levels in all strains suggesting a mitochondria-specific mechanism. Since we observed that green tea increased the lifespan of all strains tested, w1118 male flies (Lopez et al., 2014) and transferrin mutants, but had an opposing effect in mitoferrin mutants, we suggest that the lifespan extension mechanism, as well as reduction in fertility in normal male flies, by green tea is dependent on the mitochondrial iron transporter, mitoferrin, and regulation of mitochondrial iron.
MATERIALS AND METHODS
Drosophila stocks
Flies were obtained from the Bloomington Drosophila Stock Center (BDSC) at Indiana University, USA and included w1118 (BDSC# 3605), Drosophila mitoferrin, dmfrnBG00456 (BDSC# 12489), and transferrin, tsf1f05108 (BDSC# 18838). Mutant flies for mitoferrin, dmfrnBG00456, and transferrin, tsf1f05108, were developed with a w1118 background genotype.
Fly mutants with reduced expression levels, or hypomorphs, of mitoferrin and transferrin have a P-element insertion at each respective gene of interest. Hypomorph expression for Drosophila mitoferrin (dmfrn BG00456) have previously been characterized (Metzendorf and Lind, 2010). Drosophila transferrin (tsf1f05108) mutants were previously identified by the Berkeley Drosophila Genome Project (BDGP) (Bellen et al., 2004) and mutant stocks are available via BDSC. Drosophila transferrin mRNA expression has previously been characterized (Yoshiga et al., 1999).
Treatment diets and experimental conditions
All flies used for experimental assays were fed a standard Drosophila banana–molasses food diet composed of 9% carbohydrate content and 9% yeast. Control diets included a 75 μL of 9% yeast solution overlay on food, which was allowed to dry and refrigerated for at least 24 h before use. Treatment diets consisted of 10 mg/mL green tea polyphenols, purchased from LKT Laboratories, Inc. (St. Paul, MN, USA), which was added to the yeast solution. Flies were maintained at 22 ± 1 °C under a 12 h light:12 h dark cycle for all experiments. The 10 mg/mL dose was chosen based on our previous work in which a 10 mg/mL dose increased the lifespan of male w1118 flies (Lopez et al., 2014). To supplement flies with treatment diets, ~3 day old flies were loaded at ratios of 6 males and 6 females per vial. Flies were transferred to freshly treated food vials every day over the course of 10 days prior to measuring iron levels, male fertility, or gene expression.
Mitochondrial isolation and whole fly preparations
Mitochondrial isolation was performed as described by Schriner et al, (2012). Fifty flies per sample were homogenized in 2mL of ice-cold mitochondrial isolation buffer (225 mM mannitol, 75 mM sucrose, 10 mM MOPS, 1 mM EGTA, 0.5% fatty acid free BSA, PH 7.2), homogenized using a glass-Teflon dounce homogenizer and filtered through two layers of cotton gauze. A mitochondrial enriched pellet was obtained by centrifugation for 10 min at 6000 x g in 4°C. Mitochondrial pellet was re-suspended in 200 μL of lysis buffer (40 mM KCl, 25 mM Tris HCl, pH 7.5 and 1% Triton X-100) and homogenized using a hand pestle.
In preparation for total body iron measurements, whole flies were frozen in groups of 50 in centrifuge tubes. Flies were homogenized in 200 μL lysis buffer using a hand pestle. All samples were centrifuged for 10 mins at 16,000 x g in 4°C and supernatant was collected in preparation for iron measurements.
Total body and mitochondrial iron levels
Iron levels were measured using the ferrozine assay as previously described (Missirlis et al., 2006) with the modification of using 50 flies per sample. In brief, the ferrozine assay is a colorimetric assay that utilizes ascorbic acid to reduce ferric ion to the ferrous state. Ferrozine reacts with ferrous ions to form a magenta complex that absorbs at 562 nm. The absorbance is directly related to iron in the fly. Prior to iron measurements, 5 μL of supernatant was collected for protein measurements. Concentrated HCl was added to prepared samples and heated for 20 mins at 95°C. Samples were centrifuged for 2 mins at 16,000 x g in 4°C and supernatant collected. Ascorbate (75 mM), ferrozine (10 mM) and saturated ammonium acetate were added and mixed. Absorbance was read at 562nm and measurements were standardized to the amount of protein in each fly sample.
Fertility
Fertility of male flies after iron chelation was evaluated by feeding flies for 10 days varying concentrations of 0 μM, 250 μM, and 500 μM of Ethylenediaminetetraacetic acid (EDTA) (USB Corporation, Cleveland, OH, USA), an iron chelator, mixed in the yeast solution overlay. Fertility of male flies after iron supplementation was evaluated after feeding flies varying concentrations of 0 mM, 5 mM, and 10 mM ferric ammonium citrate (FAC) obtained from Sigma-Aldrich (St. Louis, MO, USA). Flies were fed treatment diets, as described above, or an equivalent control for 10 days and their fertility was measured. Male fertility was assayed by placing one treated male with one untreated virgin female per vial (n=20). Paired flies were allowed to mate for 24 hours and then transferred to new vials each day for the course of 10 days. Eggs were allowed to develop and the offspring was counted 14 days later.
Gene expressions
Quantitative PCR was performed as previously described (Schriner et al., 2012). In brief, flies were frozen in groups of 10 and RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Samples were treated with DNase (New England Biolabs, Ipswich, MA, USA) and converted to cDNA by the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Quantitative PCR was performed on a MiniOpticon real-time PCR system with SYBR green dye (Bio-Rad, Hercules, CA, USA). Relative mRNA abundances were calculated by the threshold cycle of each respective gene divided by the threshold cycle of the reference gene GAPDH (Schriner et al., 2012). Primer sequences are shown in Table 1.
Table 1.
| Gene | Primer Sequence (5′→ 3′) | Source |
|---|---|---|
| * GAPDH | F-GTTGCGGCTGAGGGCGGATT R-AGTTGATGTTGGCCGGGTCGC |
Primers were designed by NCBI/Primer-BLAST |
| dmfrn | F-TTTGCCGCCTACGAGATG R-TAGAAATGGCGTCGTGTATG |
Navarro et al, 2015 (Navarro et al., 2015) Metzendorf et al, 2009 (Metzendorf et al., 2009) |
| tsf1 | F-AAGTACTTTGGTCTGCCGGG R-GTGCCATCCTCGCACAGATA |
Primers were designed by NCBI/Primer-BLAST |
Abbreviations: F, forward; R, reverse
Reference gene
Lifespan
Flies were fed 10 mg/mL of green tea polyphenols as described above throughout their lifespan. A total of 20 vials were loaded with six males and six females in each vial (n = 120 per sex). Flies were transferred every other day to newly yeasted food and deaths were counted after each transfer until all flies died.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Fertility experiments were analyzed by Two-way ANOVA and data were presented as means ± SEM. Lifespans were analyzed by Mann-Whitney log-rank test. All other experimental data were analyzed by student t-test.
RESULTS
Iron deprivation negatively affects male fly fertility
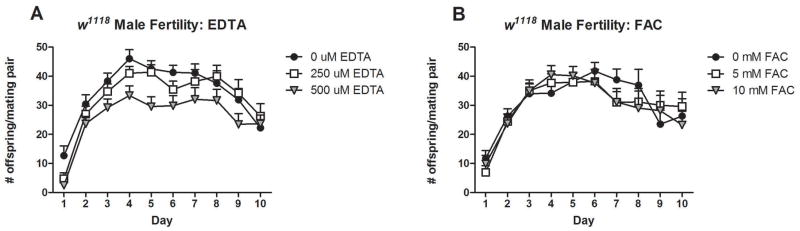
Iron has been found to be a crucial micronutrient for male Drosophila fertility and reproductive systems (Metzendorf and Lind, 2010; Sadraie and Missirlis, 2011). Experiments evaluating the interaction between iron and fertility are typically performed with mutant fly strains that exhibit sensitivity to iron availability. Here we investigated the effect of dietary iron on fly fertility in a standard laboratory strain, w1118, over a 10-day mating period. We observed that iron deprivation by an iron chelator, EDTA, decreased male fly fertility in a dose-dependent manner (P<0.0001, Fig. 1A). Iron supplementation by ferric ammonium citrate (FAC 5μM and 10μM), however, had no effect on the fertility of male flies (P>0.05, Fig. 1B).
Figure 1.
Effects of iron availability on male Drosophila fertility. Iron chelation by EDTA decreased male fertility of a standard Drosophila strain, w1118 (P< 0.0001) over a 10-day mating period (A). Iron supplementation with ferric ammonium citrate (FAC) had no effect on male fly fertility (P> 0.05) (B). Data are presented as means ± SEM per day and analyzed by Two-way ANOVA. Sample sizes for each treatment were as follows: n=18, 15, 19 for 0μM, 250uM and 500μM EDTA, respectively, and n=12, 15, 16 for 0mM, 5mM and 10mM FAC, respectively.
Green tea decreases total and mitochondrial iron levels in flies
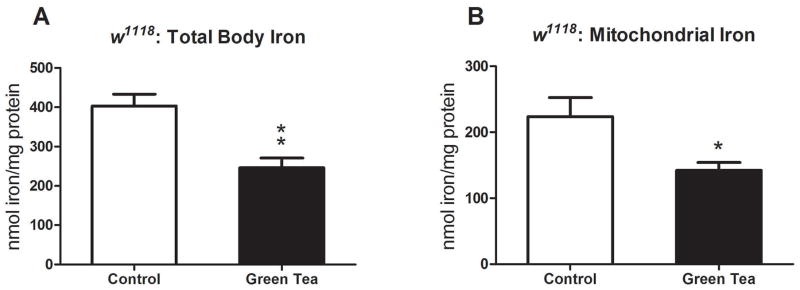
Green tea has high polyphenolic content with known iron-binding properties (Khokhar and Apenten, 2003; Perron and Brumaghim, 2009). We investigated the ability of a green tea polyphenol extract, consisting of ~80% catechin content (Lopez et al., 2016), to modulate total body iron levels. We found a 39% decrease (245.9±24.9 vs. 403.6±29.4 nmol Fe/mg protein) in total body iron compared to controls (P=0.003, Fig. 2A). Moreover, mitochondrial iron metabolism has been suggested to play a direct role in spermatogenesis of male Drosophila (Metzendorf and Lind, 2010). We, therefore, measured the iron levels in mitochondria from flies fed a diet with or without green tea. We found a 36% decrease (142.3 ±11.9 vs. 223.7±28.6 nmol Fe/mg protein) in mitochondrial iron levels from flies fed green tea versus controls (P=0.04, Fig. 2B).
Figure 2.
The impact of green tea on total body and mitochondrial iron levels in Drosophila. Green tea decreased total body iron levels in a standard Drosophila strain, w1118 (P=0.003, n=250) versus controls (A). Isolated mitochondria from flies fed green tea had reduced levels of mitochondrial iron (P=0.04, n=200) (B). Data are presented as means ± SEM and analyzed by student’s t-test.
Hypomorph mutants for mitoferrin and transferrin have increased lifespans and reduced male fly fertility
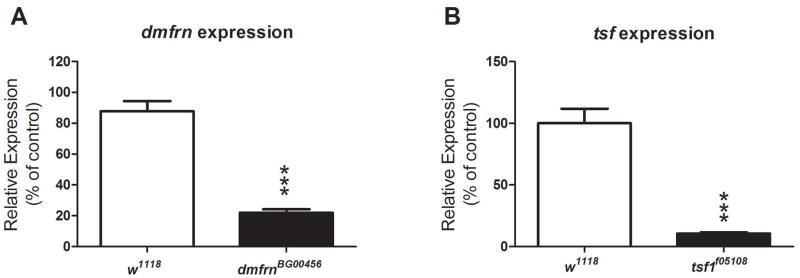
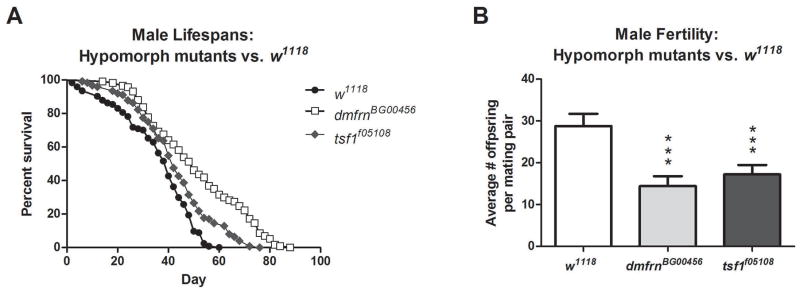
Using publicly available hypomorphs of mitoferrin and transferrin (Bellen et al., 2004; Metzendorf and Lind, 2010; Yoshiga et al., 1999), we compared their lifespan and male fertility to a standard w1118 fly strain. We first validated the mutation of dmfrnBG00456 and tsf1f05108 flies by measuring the expression levels of mitoferrin and transferrin, in each respective mutant. We confirmed a significant reduction in mitoferrin (Fig. 3A) and transferrin (Fig. 3B) expression in these mutants (P<0.0001). We then identified that mitoferrin and transferrin hypomorph mutants exhibit longer lifespans compared to w1118 (P<0.0001, Fig. 4A). Specifically, mitoferrin hypomorphs exhibited the greatest increase in lifespan (42%) followed by transferrin (19%). Baseline male fertility levels of all investigated hypomorph mutants were found to be significantly lower than that of w1118 flies (P 0.0001, Fig. 4B), 50% in mitoferrin and 40% in transferrin mutants.
Figure 3.
Background expression levels of mitoferrin and transferrin in hypomorph mutants. Mitoferrin (A) and transferrin (B) expression in dmfrnBG00456 and tsf1f05108, respectively, was found to be lower than a standard laboratory strain, w1118 (***P<0.0001). Data are presented as means ± SEM and analyzed by student’s t-test. Sample sizes were as follows: for dmfrn expression, w1118 n=40, dmfrnBG00456 n=60 and for tsf expression, w1118 n=50, tsf1f05108 n=60.
Figure 4.
Lifespan and fertility of mitoferrin and transferrin mutants compared to a standard laboratory fly strain, w1118. Hypomorph mutants, dmfrnBG00456 and tsf1f05108 exhibit longer lifespans (P<0.0001) than w1118 flies (A). Hypomorph mutants displayed reduced male fly fertility compared to w1118 (B). Lifespans were analyzed by Mantel-Cox log-rank test. For each fly strain, lifespan sample sizes, mean lifespan (means ± SEM), maximum lifespan (days) and percent increase compared to w1118 were as follows: dmfrnBG00456 n=117, 51 ± 1.7 days, 88 days, 42%; tsf1f05108 n=124, 43 ± 1.4 days, 76 days, 19%; w1118 n=124, 36 ± 1.3 days, 60 days. Fertility experiments were analyzed by student’s t-test and data presented as means ± SEM, ***P≤0.0001, n=20 mating pairs.
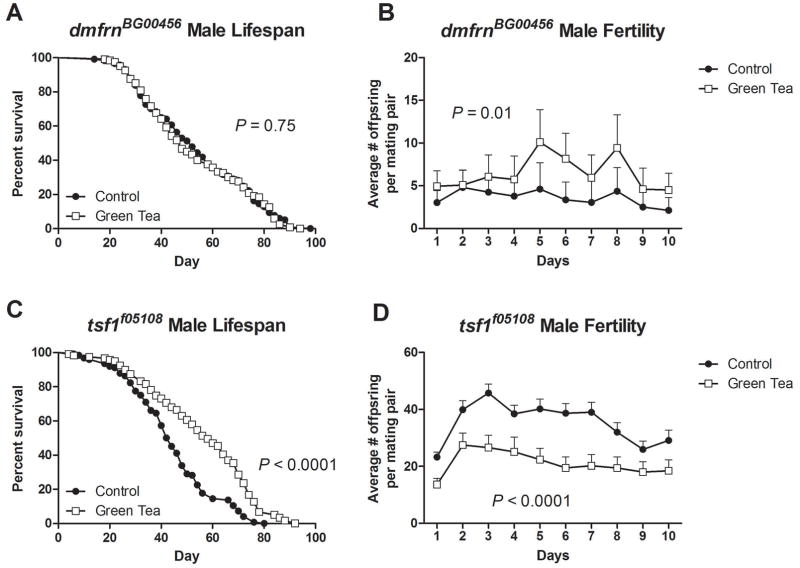
Green tea has no effect on lifespan, but increases male fly fertility of mitoferrin mutants
Previously we reported that green tea increased male Drosophila lifespan by 19% in w1118 flies while reducing fertility (Lopez et al., 2014). Here we investigated the effect of green tea on the lifespan and male fertility of hypomorph mutants, mitoferrin and transferrin. We identified that green tea could no longer increase the lifespan of mitforerrin hypomorph mutants (P>0.05, Fig. 5A) but did increase male fertility (P=0.01, Fig. 5B). Interestingly, transferrin mutants exhibited an increase in lifespan with supplementation of green tea (P<0.0001, Fig. 5C). However, green tea did have a negative effect on male fly fertility of transferrin hypomorph mutants (P<0.0001, Fig. 5D). The increase in lifespan and decrease in fertility by green tea in transferrin mutant flies are similar to those observed in previous reports with w1118 [1].
Figure 5.
The impact of green tea on lifespan and fertility of mitoferrin and transferrin mutants. Green tea had no effect on dmfrnBG00456 hypomorph mutant lifespan (A) but increased male fly fertility (B). Green tea increased the lifespan of tsf1f05108 mutants (C) however exhibited a decline in male fertility (D). Lifespans were analyzed by Mantel-Cox log-rank test. Sample sizes for lifespans, control and green tea respectively, were as follows: dmfrnBG00456 n=117,120; tsf1f05108 n=124,119. Fertility experiments were analyzed by Two-way ANOVA and data are presented as means ± SEM per day, n=20 mating pairs.
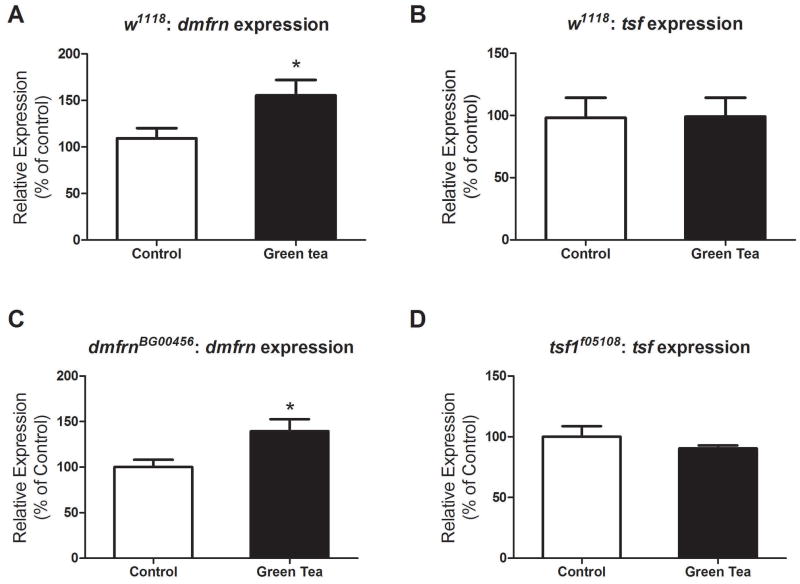
Green tea up-regulates the expression of mitoferrin, but not transferrin, in hypomorph mutants and w1118 flies
To determine whether green tea has any effect on the expression levels of iron metabolizing genes, we compared the levels of mitoferrin and transferrin in a standard laboratory fly strain, w1118. We showed that green tea increased the expression of mitoferrin (P=0.04, Fig. 6A) while no difference was detected with transferrin expression levels (P>0.05, Fig. 6B). Subsequently, since hypomorph mutants have residual expression for their affected genes (Fig. 3), we questioned whether green tea acts by modulating their expression. We showed that similar to w1118 flies green tea up-regulated the expression of mitoferrin in mitoferrin hypomorph mutants (P=0.04, Fig. 6C). Green tea had no effect on the expression levels of transferrin in transferrin mutants (P>0.05, Fig. 6D).
Figure 6.
The effect of green tea on mitoferrin and transferrin expression in w1118 and hypomorph mutants. Green tea up-regulated expression of mitoferrin (A) (P=0.04, n=50 per treatment) in w1118 flies but had no effect on the expression levels of transferrin (B) (P>0.05, control n=60, green tea n=50). Green tea up-regulated mitoferrin in dmfrnBG00456 hypomorph mutants (C) (P=0.04, n=50 per treatment). Green tea did not affect transferrin expression levels in tsf1f05108 flies (D) (P>0.05, control n=60, green tea n=50). Data are represented as means ± SEM and analyzed by student’s t-test.
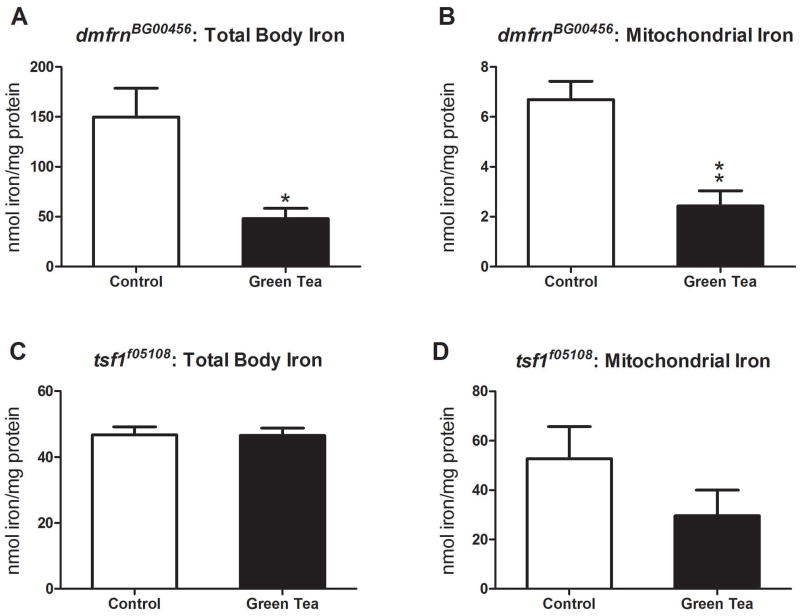
Green tea decreases total body and mitochondrial iron levels in mitoferrin but not transferrin hypomorph mutants
Since green tea increased the expression levels of the mitochondrial iron importer, mitoferrin, we evaluated the effect of green tea on total body and mitochondrial iron levels in hypomorph mutants. We found that green tea reduced total body and mitochondrial iron levels by 68% and 65%, respectively, in mitoferrin hypomorphs, (P<0.05, Fig. 7A and P<0.005, Fig. 7B). Green tea had no effect on total body iron levels in transferrin mutants (P>0.05, Fig. 7C) and did not significantly reduce mitochondrial iron (P>0.05, Fig. 7D).
Figure 7.
The effect of green tea on total body and mitochondrial iron levels in mitoferrin and transferrin mutants. Green tea significantly reduced total body (A) and mitochondrial iron levels (B) in dmfrnBG00456 hypomorph mutants (*P<0.05 and **P<0.005, respectively, n=50 per treatment). Green tea did not affect total body iron levels (C) nor mitochondrial iron levels (D) of transferrin mutants (P>0.05, n=40 per treatment). Data are represented as means ± SEM and analyzed by student’s t-test.
DISCUSSION
We and others have previously reported the ability of green tea and its primary active flavonoid, EGCG, to increase the lifespan of different strains of Drosophila melanogaster (Jimenez-Del-Rio et al., 2010; Li et al., 2007; Lopez et al., 2014; Massie et al., 1993; Wagner et al., 2015). In this study we found that the mechanism of green tea’s action on male Drosophila lifespan and fertility involves iron regulators, such as mitoferrin. Over the years, the mechanism of action of green tea has been evaluated extensively. Massie et al. (1993) had first suggested that green tea induced lifespan extension in Drosophila by its ability to inhibit iron absorption and thus iron accumulation throughout life (Massie et al., 1993). With a number of studies reporting green tea’s iron binding activity, some studies have linked the importance of iron to male Drosophila spermatogenesis and fertility (Metzendorf and Lind, 2010). Specifically, Metzendorf and Lind (2010) showed that iron chelation in the diet increased sterility in mitoferrin hypomorph mutants whereas iron supplementation improved fertility demonstrating the importance of iron for spermatogenesis. To evaluate the interaction between iron and fertility we tested whether iron deprivation in the diet had any effects on male fertility in normal w1118 flies. We too identified a reduction in male fertility when iron levels were reduced by an iron chelator, EDTA. Next, we tested whether iron supplementation had any effects on male fertility. Our results did not show any improvements in male fertility with iron supplementation. We surmised that in normal flies, which display typical reproductive abilities and thus functional iron metabolizing pathways, the addition of iron in the diet would have no additive effect on fertility. In healthy flies, excess iron would be stored in ferritin, an iron-storage protein, and iron can be secreted from this storage protein to the gut lumen during iron over-load conditions (Mandilaras et al., 2013). We further explored the levels of iron in whole flies and in fly mitochondria after treatment with green tea polyphenols and identified a significant reduction of iron levels in both homogenates, 39% and 36% reduction, respectively. The reduction in mitochondrial iron is of particular interest since mitochondrial iron involving the iron transporter, mitoferrin, has a direct role in the development of sperm. Interestingly, green tea’s primary catechin, EGCG, has been reported to accumulate in the mitochondria of neuronal cells (Schroeder et al., 2009). In accordance with this finding, it is thus likely that the presence of green tea catechins in the mitochondria can result in reduced mitochondrial iron levels.
While the reduction in iron levels is a direct consequence of green tea supplementation and hence reduction in male fertility, the reported effects of green tea selectively increasing male flies’ lifespan (Li et al., 2007; Lopez et al., 2014; Massie et al., 1993; Wagner et al., 2015) could be a secondary effect of the treatment. It is well established that impairments in reproductive abilities, such as a decrease in egg laying for females (Flatt, 2011; Kirkwood and Rose, 1991) or decrease in sperm production for males (Prowse and Partridge, 1997), have an inverse relationship with fruit fly lifespan. However, it is likely that the reduction of iron has physiological effects unrelated to reproduction, some of which could result in an increased lifespan. In this study we proposed that the reduction in fertility was caused by the reduction in mitochondrial iron levels, which is critical for spermatogenesis (Metzendorf and Lind, 2010). This decrease in fertility then contributed, at least in part, to an increase in male fly lifespan (Lopez et al., 2014).
To evaluate whether green tea polyphenols act through the regulation of iron metabolism and hence in turn affect fertility and lifespan, we utilized mutant flies with deficiencies in their ability to regulate iron. Flies with defects in Drosophila mitoferrin and transferrin were specifically chosen as these proteins display a diverse involvement with iron including the transport of iron into the mitochondria, mitoferrin, or throughout the fly, transferrin. We first established that these mutants exhibit longer lifespans and reduced fertility than normal flies. This supports the notion that these iron-regulators follow an inverted relationship with lifespan and fertility. Drosophila mitoferrin mutants, have previously been found to exhibit a reduced male fertility phenotype (Metzendorf and Lind, 2010). However, the lifespan and fertility phenotypes for transferrin mutant flies have not yet been characterized. One study, which used C. elegans to down-regulate the expression of mitoferrin, identified a 50–80% increase in worm lifespan, as well as abnormal developmental phenotypes and reduced production of progeny (Ren et al., 2012). This observation in C. elegans is interesting since we previously reported that green tea polyphenols exhibited similar effects on lifespan, development and fertility in Drosophila (Lopez et al., 2014; Lopez et al., 2016). It is therefore plausible that green tea polyphenols require mitoferrin to affect lifespan. Since transferrin hypomorph mutants also exhibited an increase in lifespan and reduced fertility, it was also of interest to determine whether green tea required transferrin as well.
The requirement of transferrin was evaluated through hypomorph mutant flies with a dysfunction in the transferrin gene. We observed that green tea increased the lifespan and reduced male fertility of transferrin hypomorph mutants, a result that parallels green tea treatment in normal w1118 flies (Lopez et al., 2014). In addition, treatment with green tea showed no change in transferrin expression levels. This led us to conclude that green tea polyphenols do not require transferrin to increase Drosophila lifespan. This result is not surprising since green tea catechins are known to bind to non-transferrin bound iron (NTBI) (Thephinlap et al., 2007) and thus act independently of the action of transferrin proteins.
Mitoferrin hypomorph mutants under the treatment of green tea, however, exhibited no increase in lifespan and instead experienced an increase in male fertility. To begin with, mitoferrin flies exhibit significantly decreased fertility which may explain their increased lifespans. Green tea polyphenols, which block iron uptake in w1118 and mitoferrin mutant flies, resulted in an up-regulation of mitoferrin expression, likely to compensate for low iron levels. This resulted in a moderate increase in fertility, which was insufficient in magnitude to affect lifespan.
Since green tea did not increase the lifespan of mitoferrin hypomorph mutants, blocked iron uptake, and up-regulated the expression levels of mitoferrin in both hypomorph mutants and normal flies, green tea polyphenols may require mitoferrin, and specifically the reduction of mitochondrial iron, to increase the lifespan of Drosophila melanogaster. As for green tea’s ability to specifically increase male fly lifespan while negatively affecting the fertility of normal flies, our results suggest that green tea’s iron-binding properties are responsible for the unique interplay between the regulation of iron metabolizing proteins, male fly fertility, and lifespan.
Acknowledgments
Special thanks to Ms. Julia Barbour and Mr. Cristian Gonzalez for their assistance with stock maintenance and iron measurements. This study was supported in part by UCI Bridge Funds and the National Institutes of Health, grant R21AT004987.
References
- Abbas S, Wink M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009;75(3):216–221. doi: 10.1055/s-0028-1088378. [DOI] [PubMed] [Google Scholar]
- Arruda LF, Arruda SF, Campos NA, de Valencia FF, Siqueira E. Dietary iron concentration may influence aging process by altering oxidative stress in tissues of adult rats. PloS One. 2013;8(4):e61058. doi: 10.1371/journal.pone.0061058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM. The BDGP gene disruption project single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167(2):761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettedi L, Aslam MF, Szular J, Mandilaras K, Missirlis F. Iron depletion in the intestines of Malvolio mutant flies does not occur in the absence of a multicopper oxidase. J Exp Biol. 2011;214(6):971–978. doi: 10.1242/jeb.051664. [DOI] [PubMed] [Google Scholar]
- Flatt T. Survival costs of reproduction in Drosophila. Exp Gerontol. 2011;46(5):369–375. doi: 10.1016/j.exger.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Folwell JL, Barton CH, Shepherd D. Immunolocalisation of the D. melanogaster Nramp homologue Malvolio to gut and Malpighian tubules provides evidence that Malvolio and Nramp2 are orthologous. J Exp Biol. 2006;209(10):1988–1995. doi: 10.1242/jeb.02193. [DOI] [PubMed] [Google Scholar]
- Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochimica et Biophysica Acta-Gen Subj. 2012;1820(3):188–202. doi: 10.1016/j.bbagen.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Hales KG. Iron testes: sperm mitochondria as a context for dissecting iron metabolism. BMC Biol. 2010;8:79. doi: 10.1186/1741-7007-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Del-Rio M, Guzman-Martinez C, Velez-Pardo C. The effects of polyphenols on survival and locomotor activity in Drosophila melanogaster exposed to iron and paraquat. Neurochem Res. 2010;35(2):227–238. doi: 10.1007/s11064-009-0046-1. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Apenten RKO. Iron binding characteristics of phenolic compounds: some tentative structure–activity relations. Food Chem. 2003;81(1):133–140. [Google Scholar]
- Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Phil Trans of the Royal Society B: Bio Sci. 1991;332(1262):15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontol. 2007;8(5):567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- Li YM, Chan HY, Huang Y, Chen ZY. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol Nutr Food Res. 2007;51(5):546–554. doi: 10.1002/mnfr.200600238. [DOI] [PubMed] [Google Scholar]
- Lopez T, Schriner SE, Okoro M, Lu D, Chiang BT, Huey J, Jafari M. Green tea polyphenols extend the lifespan of male Drosophila melanogaster while impairing reproductive fitness. J Med Food. 2014;17(12):1314–1321. doi: 10.1089/jmf.2013.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez TE, Pham HM, Barbour J, Tran P, Van Nguyen B, Hogan SP, Homo RL, Coskun V, Schriner SE, Jafari M. The impact of green tea polyphenols on development and reproduction in Drosophila melanogaster. J Functional Foods. 2016;20:556–566. doi: 10.1016/j.jff.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JC. Potential role of green tea catechins in various disease therapies: progress and promise. Clin Exp Pharmacol Physiol. 2012;39(3):265–273. doi: 10.1111/j.1440-1681.2012.05673.x. [DOI] [PubMed] [Google Scholar]
- Mandilaras K, Pathmanathan T, Missirlis F. Iron absorption in Drosophila melanogaster. Nutrients. 2013;5(5):1622–1647. doi: 10.3390/nu5051622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Williams TR. Inhibition of iron absorption prolongs the life span of Drosophila. Mech Ageing Dev. 1993;67(3):227–237. doi: 10.1016/0047-6374(93)90001-8. [DOI] [PubMed] [Google Scholar]
- Metzendorf C, Lind M. Mitoferrin is essential for normal development in Drosophila. Amer J Hematol. 2013;88:E76–E77. [Google Scholar]
- Metzendorf C, Lind MI. Drosophila mitoferrin is essential for male fertility: evidence for a role of mitochondrial iron metabolism during spermatogenesis. BMC Dev Biol. 2010;10:68. doi: 10.1186/1471-213X-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzendorf C, Wu W, Lind M. Overexpression of Drosophila mitoferrin in l (2) mbn cells results in dysregulation of Fer1HCH expression. Biochem J. 2009;421:463–471. doi: 10.1042/BJ20082231. [DOI] [PubMed] [Google Scholar]
- Missirlis F, Holmberg S, Georgieva T, Dunkov BC, Rouault TA, Law JH. Characterization of mitochondrial ferritin in Drosophila. Proc Natl Acad Sci U S A. 2006;103(15):5893–5898. doi: 10.1073/pnas.0601471103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JA, Botella JA, Metzendorf C, Lind MI, Schneuwly S. Mitoferrin modulates iron toxicity in a Drosophila model of Friedreich s ataxia. Free Radic Biol Med. 2015;85:71–82. doi: 10.1016/j.freeradbiomed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005;202(2):199–211. doi: 10.1016/j.taap.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53(2):75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- Prowse N, Partridge L. The effects of reproduction on longevity and fertility in male Drosophila melanogaster. J Insect Physiol. 1997;43(6):501–512. doi: 10.1016/s0022-1910(97)00014-0. [DOI] [PubMed] [Google Scholar]
- Ren Y, Yang S, Tan G, Ye W, Liu D, Qian X, Ding Z, Zhong Y, Zhang J, Jiang D. Reduction of mitoferrin results in abnormal development and extended lifespan in Caenorhabditis elegans. PloS One. 2012;7(1):e29666–e29666. doi: 10.1371/journal.pone.0029666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadraie M, Missirlis F. Evidence for evolutionary constraints in Drosophila metal biology. Biometals. 2011;24(4):679–686. doi: 10.1007/s10534-011-9420-y. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Katoozi NS, Pham KQ, Gazarian M, Zarban A, Jafari M. Extension of Drosophila lifespan by Rosa damascena associated with an increased sensitivity to heat. Biogerontol. 2012;13(2):105–117. doi: 10.1007/s10522-011-9357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder EK, Kelsey NA, Doyle J, Breed E, Bouchard RJ, Loucks FA, Harbison RA, Linseman DA. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antiox Redox Signal. 2009;11(3):469–480. doi: 10.1089/ars.2008.2215. [DOI] [PubMed] [Google Scholar]
- Sinija VR, Mishra HN. Green tea: Health benefits. J Nutri Environ Med. 2008;17(4):232–242. [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68(1):6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Miyoshi N, Isemura M. Health-promoting effects of green tea. Proc Japan Academy, Series B. 2012;88(3):88–101. doi: 10.2183/pjab.88.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Zhou B. Iron homeostasis in insects: Insights from Drosophila studies. IUBMB life. 2013;65(10):863–872. doi: 10.1002/iub.1211. [DOI] [PubMed] [Google Scholar]
- Thephinlap C, Ounjaijean S, Khansuwan U, Fucharoen S, Porter J, Srichairatanakool S. Epigallocatechin-3-gallate and epicatechin-3-gallate from green tea decrease plasma non-transferrin bound iron and erythrocyte oxidative stress. Med Chem. 2007;3(3):289–296. doi: 10.2174/157340607780620608. [DOI] [PubMed] [Google Scholar]
- Tvrda E, Peer R, Sikka SC, Agarwal A. Iron and copper in male reproduction: a double-edged sword. J Assist Reproduc Genetics. 2015;32(1):3–16. doi: 10.1007/s10815-014-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura N, Rea S, Henderson ST, Condo I, Johnson TE, Testi R. Reduced expression of frataxin extends the lifespan of Caenorhabditis elegans. Aging Cell. 2005;4(2):109–112. doi: 10.1111/j.1474-9726.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Wagner AE, Piegholdt S, Rabe D, Baenas N, Schloesser A, Eggersdorfer M, Stocker A, Rimbach G. Epigallocatechin gallate affects glucose metabolism and increases fitness and lifespan in Drosophila melanogaster. Oncotarget. 2015;6(31):30568–30578. doi: 10.18632/oncotarget.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb O, Mandel S, Amit T, Youdim MB. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J Nutri Biochem. 2004;15(9):506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Xu J, Marzetti E, Seo AY, Kim J-S, Prolla TA, Leeuwenburgh C. The emerging role of iron dyshomeostasis in the mitochondrial decay of aging. Mech Ageing Dev. 2010;131(7):487–493. doi: 10.1016/j.mad.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiga T, Georgieva T, Dunkov BC, Harizanova N, Ralchev K, Law JH. Drosophila melanogaster transferrin. Euro J Biochem. 1999;260(2):414–420. doi: 10.1046/j.1432-1327.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78(18):2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]