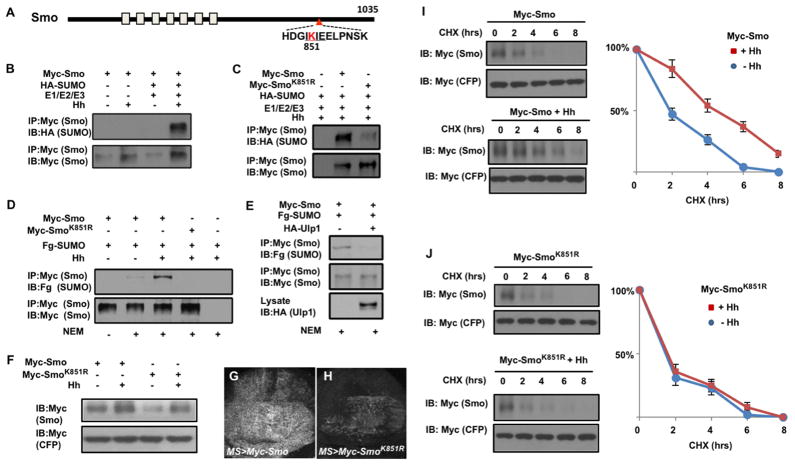
Figure 3. Hh stimulates sumoylation of Smo at K851 to regulate its abundance.
(A) Schematic drawing of Smo. (B–C) Western blots to detect SUMO conjugated Smo derived from S2 cells transfected with Myc-Smo (B, C) or Myc-SmoK851R (C) together with HA-SUMO and the SUMO E1/E2/E3 enzymes in the absence or presence of Hh-conditioned medium. (D–E) Western blots to detect SUMO conjugated Smo derived from S2 cells transfected with Myc-Smo or Myc-SmoK851R and Fg-SUMO in the absence or presence of Hh-conditioned medium (D) or HA-Ulp1 cotransfection (E). (F) Western blot analysis of cell extracts from S2 cells transfected with either Myc-Smo or Myc-SmoK851R and treated with Hh-conditioned or control medium. (G–H) Wing discs expressing Myc-Smo (G) or Myc-SmoK851R (H) under the control of MS1096 in conjunction with Gal80ts. Larvae were grown at 18°C until late third instar, then shifted to 30°C for 16 hours before immunostained with anti-Myc antibody. (I–J) Protein stability assay of Myc-Smo and Myc-SmoK851R transfected into S2 cells. Quantification of Myc-Smo levels at different time points was shown to the right. Data are mean ± SD from three independent experiments. Signal intensities at t=0 were defined as 100%. Of note, to ensure relatively equal level of Smo protein at t=0, more Myc-SmoK851R DNA than that of Myc-Smo was transfected and more DNA was transfected in the absence of Hh treatment compared with that treated with Hh-conditioned medium. See also Figure S3.