Abstract
Toxoplasmosis, a benign infection, is asymptomatic or paucisymptomatic in over 80% of cases, except in immunocompetent patients suffering from ocular toxoplasmosis or in immunocompromised patients with opportunistic or congenital toxoplasmosis. Diagnosis is based mainly on serology testing. Thus, we compared the performance of the nine most commonly used commercial automated or semiautomated immunoassays for IgG and IgM Toxoplasma gondii antibody detection, that is, the Advia Centaur, Architect, AxSYM, Elecsys, Enzygnost, Liaison, Platelia, VIDAS, and VIDIA assays. The assays were conducted on four panels of serum samples derived during routine testing from patients with an interfering disease and who exhibited a low IgG antibody level in one of two clinical settings, namely, acute or chronic toxoplasmosis. As a result, IgG sensitivities ranged from 97.1% to 100%, and IgG specificities ranged from 99.5% to 100%. For IgG quantification, strong differences in IgG titers (expressed in IU/ml) were noted depending on the assay used. IgM sensitivities ranged from 65% to 97.9%, and IgM specificities ranged from 92.6% to 100%. For defining the best serological strategies to be implemented, it appears crucial to compare the diagnostic performance of the different tests with respect to their specificity and sensitivity in detecting the presence of IgG and IgM antibodies.
INTRODUCTION
Toxoplasmosis, a benign infection that is asymptomatic or paucisymptomatic in more than 80% of cases (1, 2), is caused by a cosmopolite protozoan parasite, Toxoplasma gondii. The prevalence of T. gondii infection varies from one country to another, depending on environmental conditions and eating habits. In the United States, Great Britain, and southeastern Asia, the T. gondii infection prevalence is less than 30% (3, 4), whereas it exceeds 60% in Africa and Latin America (5). In France, its prevalence decreased over the past decades from 83% in 1965 to 37% in 2010 (6, 7).
Infection that occurs during pregnancy in a noninfected woman may result in congenital toxoplasmosis in the child (8). In immunosuppressed patients (HIV-infected or transplant patients), chronic infection or its reactivation can also lead to severe toxoplasmosis and can even be lethal if not successfully treated (9). Ocular toxoplasmosis may be observed in immunocompetent patients (10). The diagnosis of toxoplasmosis is based mainly, but not only, on serological tests for IgG and IgM (11, 12). These screening techniques enable the immunological status to be defined and the exposure risk for seronegative subjects to be estimated. They also allow us to implement prevention programs and to assess the impact of the disease on public health.
In pregnant women, assessing IgG and IgM levels enables differentiation between acute and chronic infections and confirmation of the absence of previous exposure (13). In clinical situations in which the date of the T. gondii infection is difficult to establish, Toxoplasma IgG avidity testing can be performed (14–16). Since 2006, the French National Reference Center for Toxoplasmosis (NRCT) has been entrusted with evaluation of the methods used for the serological diagnosis of toxoplasmosis (11, 12) in accordance with national recommendations (17).
Thus, in this study, we sought to evaluate and compare the performance of the most commonly used commercial automated or semiautomated immunoassays for IgG and IgM T. gondii antibody detection that are available in Europe. Such a comparison appears crucial for defining the best serological strategies to be implemented, depending on the specificity and sensitivity of the different tests in detecting IgG and IgM antibodies (18).
MATERIALS AND METHODS
Samples.
The serum samples were supplied by members of the NRCT network, and the presence of IgG or IgM antibodies was confirmed based on the results of the reference technique using at least two different methods for each antibody isotype, namely, the indirect immunofluorescence assay (IFAT-G) (19) and dye test (20–22) for IgG detection, and IFAT-M and immunosorbent agglutination assay (ISAGA-M; bioMérieux SA, Marcy-l'Etoile, France) (23) for IgM detection. IgA levels were not assessed. The serum samples were subsequently divided into four panels with the objective to analyze 10 assays distributed into one of two assessment groups: assessment 1 or assessment 2.
Panel 1 containing sera from routine testing was employed to estimate the tests' sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). This panel comprised negative sera, sera positive for IgG yet negative for IgM, and sera positive for both IgG and persistent IgM. For assessment 1, panel 1 contained 419 serum samples distributed as follows: 199 serum samples were negative, 174 positive for IgG yet negative for IgM (62 samples with an IgG titer of <50 IU/ml by immunofluorescence of IgG, 48 with an IgG titer between 50 and 100 IU/ml, 43 with an IgG titer between 100 and 200 IU/ml, and 25 with an IgG titer of >200 IU/ml), and 45 were positive for both IgG and IgM. For assessment 2, panel 1 contained 406 serum samples distributed as follows: 199 samples were negative, 169 were positive for IgG yet negative for IgM (48 samples with an IgG titer of <50 IU/ml, 49 with an IgG titer between 50 and 100 IU/ml, 46 with an IgG titer between 100 and 200 IU/ml, and 26 with an IgG titer of >200 IU/ml), and 39 were positive for both IgG and IgM.
Panel 2 was designed to evaluate clinical specificity. This panel comprised sera from patients with potentially interfering diseases, namely, autoimmune diseases such as scleroderma diagnosed by ELISA or immunofluorescence tests, such as those for antinuclear antibodies (ANA), viral infections (including hepatitis A [HA], herpes, and cytomegalovirus [CMV] diseases), and bacterial infections (including Lyme disease and syphilis). Among these serum samples, 46 were used for assessment 1, and 55 were used for assessment 2.
Panel 3 was aimed at assessing the assays' detectability and specificity with sera taken from patients with a low IgG level, without IgM, and classified by at least one network member in the gray zone by means of at least one of the two methods employed. This was subsequently confirmed by the detection of an equivocal or low IgG titer when using the dye test. Of these serum samples, 14 were used for assessment 1, and 45 were used for assessment 2.
Panel 4 sought to evaluate the different methods in clinical settings, such as acute or chronic infections based on patient follow-up (24). This panel comprised sera from acute toxoplasmosis patients (seroconversion confirmed by a previously negative serum sample), with 15 patients' sera used for assessment 1 and 16 samples for assessment 2. Panel 4 also comprised sera from 19 chronically infected (>1 year) patients with persistent positive IgM levels that were used for assessment 1 and sera from 42 patients used for assessment 2.
Assays.
Overall, 10 registered kits with European Conformity (CE) marking were evaluated. The AxSYM Toxo IgM and IgG assay (Abbott, Wiesbaden, Germany) is based on microparticle enzyme immunoassay (MEIA) technology (25, 26).
The Access and Toxo Enzygnost Toxoplasmose assays (Siemens Healthcare Diagnostics, Deerfield, IL, USA) (27) rely on immunoenzymatic technology (i.e., enzyme immunoassays [EIAs]) technology for IgG or enzyme-linked fluorescent assays (ELFAs) for VIDAS and immunocapture assays for IgM.
The Architect (Abbott) (28), Advia Centaur (Siemens, USA) (29), and Liaison (DiaSorin, Saluggia, Italy) (30) Toxo IgG and IgM assays are based on chemiluminescent microparticle immunoassay (CMIA) technology.
The Elecsys Toxo IgG and IgM assay (Roche Diagnostics GmbH, Penzberg, Germany) relies on electrochemiluminescence immunoassays (ECLIAs) (31).
The Platelia Toxo IgG and IgM (Bio-Rad, Marnes-la-Coquette, France) assay is based on immunoenzymatic technology (i.e., enzyme immunoassays [EIAs]) (32).
The VIDIA Toxo system (bioMérieux, Marcy-l'Etoile, France) combines a two-step immunoassay for IgG and immunocapture method for IgM with paramagnetic microparticles and final chemiluminescence detection (33).
The results are expressed in strict accordance with manufacturer instructions (see Table S1 in the supplemental material).
Due to the small amount of serum available from each patient sample, we divided the evaluations into two assessment groups. In assessment 1, we analyzed the performance of the AxSYM, VIDAS, and VIDIA assays, and the performances of the remaining assays were analyzed in assessment 2.
A specificity of ≥99% was required to continue the evaluation on the specific panel or panel 2.
RESULTS
Performance of IgG detection.
During the course of this study, assessment of the Access assay was not pursued owing to its poor specificity on panel 1, where we found a 90% specificity, which is not in accordance with the previously set requirement of >99%.
The working group of our network chose a 99% specificity for two reasons. First, each of the evaluated assays was supposed to exhibit a 99% specificity according to the manufacturer's notice. Second, specificity appears to be crucial given that high specificity would prevent false-positive results, which would likely bring an end to the serological follow-up and hygienic-dietetic measures. In such a context, pregnant women may be exposed to the parasite, with nonsymptomatic toxoplasma infection.
Of the 199 serum samples that were negative for both IgG and IgM, 19 were actually positive for IgG (9.5%). Following serum centrifugation, the specificity reached 99.5%. It should be noted, however, that a requirement for precentrifugation was not mentioned in the provider's instructions. Thus, the risk of false positives should not be overlooked. The provider was informed about the issue, and the assessment of this assay was discontinued.
Clinical sensitivities of >99.5% for IgG detection were observed with the Elecsys, Advia Centaur, AxSYM, Architect, VIDAS, and VIDIA assays (Table 1), and lower sensitivities were observed with the Enzygnost, Platelia, and Liaison assays (98.1%, 97.2%, and 95.8%, respectively). Since the IgG specificity was at least 99.5% for the nine assays, assessment pursuance was validated. All of the PPVs were higher than 99.5%, while three assays (Liaison, Platelia, and Enzygnost) exhibited NPVs of less than 99.5% (95.7%, 97.1%, and 98.3%, respectively).
TABLE 1.
Performance comparison of the nine assays for T. gondii-specific IgG antibody detection in routine panels (panel 1) containing 419 serum samples for assessment 1 and 406 serum samples for assessment 2
| Assay | Performance (%) |
|||
|---|---|---|---|---|
| Sensitivitya | Specificityb | PPV | NPV | |
| Advia Centaurc | 100 | 100 | 100 | 100 |
| Architectc | 99.6 | 99.5 | 99.6 | 99.5 |
| AxSYMd | 99.6 | 99.5 | 99.6 | 99.5 |
| Elecsysc | 100 | 100 | 100 | 100 |
| Enzygnostc | 98.1 | 100 | 100 | 98.3 |
| Liaisonc | 95.8 | 99.5 | 99.5 | 95.7 |
| Plateliac | 97.2 | 100 | 100 | 97.1 |
| VIDASd | 99.6 | 100 | 100 | 99.5 |
| VIDIAd | 99.6 | 100 | 100 | 99.5 |
Sensitivity = true positive/(true positive + false negative).
Specificity = true negative/(true negative + false positive).
Assessment 2.
Assessment 1.
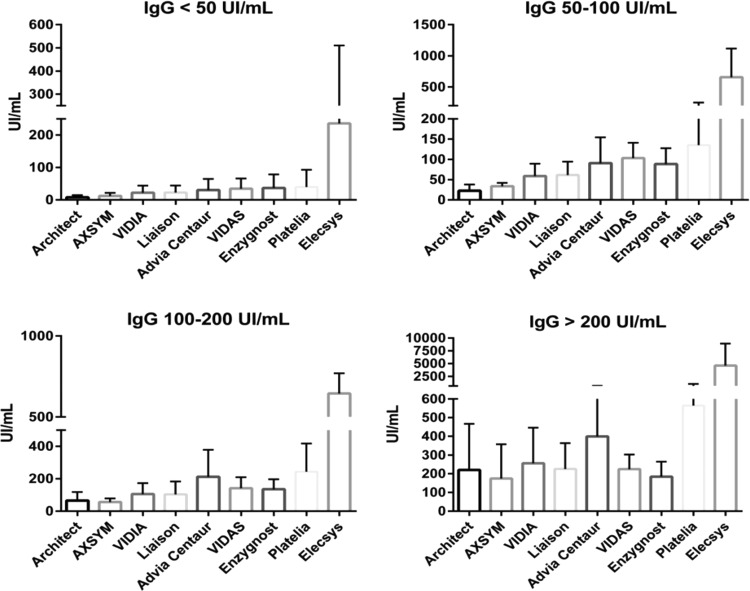
Depending on the assay, strong differences in IgG titers (expressed in IU/ml) were noted between the IFAT-G and dye test methods (Table 1 and Fig. 1). For sera with a titer of less than 50 IU/ml, the median IgG levels varied from 5.6 IU/ml with the Architect assay to 1,450.5 IU/ml with the Elecsys assay. The other assays had a titer between 10 and 30 IU/ml.
FIG 1.
Comparison of IgG titers (UI/ml) expressed as means ± standard deviation of data from the nine assays in the routine (panel 1) and chronic infection (>1 year; panel 4) panels.
Concerning sera with a titer ranging from 50 to 100 IU/ml, the lowest titer (18.4 IU/ml) was obtained with the Architect assay, and the highest titer (282 IU/ml) was obtained with the Elecsys assay. The other titers ranged from 30 to 100 IU/ml for the assays tested. Concerning the sera with a titer between 100 and 200 IU/ml, a median titer of 38.5 IU/ml was observed with the Architect assay and a median titer of 1,450.5 IU/ml was observed with the Elecsys assay. The titer of most assays was between 50 and 200 IU/ml. Regarding the serum samples with the highest titers (>200 IU/ml), the lowest median was measured at 123.5 IU/ml (AxSYM assay), and the highest titer was observed at 3,292 IU/ml (Elecsys assay). The other assays had a median titer between 140 and 400 IU/ml.
Concerning sera from chronic (>1 year) toxoplasmosis patients with residual IgM levels, median IgG levels were measured at 22.9 IU/ml and 357.2 IU/ml for the Architect and Elecsys assays, respectively (Table 2).
TABLE 2.
Detailed comparison of quantitative IgG levels when using the nine assays analyzed in routine panels (panel 1)
| Result category | IgG level (IU/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Advia Centaura | Architecta | AXSYMb | Elecsysa | Enzygnosta | Liaisona | Plateliaa | VIDASb | VIDIAb | |
| <50 IU/ml | |||||||||
| Mean | 23.5 | 7.9 | 13.3 | 174.7 | 36.7 | 25.9 | 40.1 | 36.5 | 23.6 |
| Median | 13.7 | 5.6 | 10.1 | 108.1 | 27.5 | 17.1 | 28.0 | 21.0 | 16.5 |
| SD | 27.2 | 7.0 | 9.6 | 181.5 | 41.3 | 18.8 | 53.1 | 32.1 | 22.2 |
| 95% CIc | 7.7 | 1.9 | 2.4 | 51.3 | 11.7 | 5.3 | 15 | 7.6 | 5.2 |
| 50–100 IU/ml | |||||||||
| Mean | 90.8 | 22.6 | 33.6 | 339.3 | 88.5 | 61.5 | 135.2 | 103.4 | 58.8 |
| Median | 78.5 | 18.4 | 32.0 | 282.0 | 86.0 | 54.2 | 136.0 | 99.0 | 51.0 |
| SD | 62.9 | 15.3 | 8.4 | 190.4 | 38.6 | 32.4 | 113.8 | 37.6 | 30.3 |
| 95% CI | 18 | 4 | 2.3 | 53 | 11 | 9 | 32 | 11.2 | 9 |
| 100–200 IU/ml | |||||||||
| Mean | 212.1 | 65.5 | 55.9 | 1820.8 | 135.3 | 104.2 | 243.4 | 142.1 | 105.4 |
| Median | 171.5 | 38.5 | 50.2 | 1450.5 | 130.0 | 85.1 | 225.0 | 134.0 | 87.0 |
| SD | 167.0 | 53.2 | 23.0 | 1526.7 | 61.8 | 79.2 | 174.3 | 67.0 | 68.4 |
| 95% CI | 40 | 16.6 | 6.8 | 54.8 | 19.4 | 22.5 | 37.8 | 19.8 | 21.4 |
| >200 IU/ml | |||||||||
| Mean | 399.7 | 220.4 | 174.3 | 4,605.4 | 184.6 | 225.6 | 564.8 | 225.0 | 256.3 |
| Median | 327.8 | 144.2 | 123.5 | 3,292.0 | 195.0 | 236.0 | 386.5 | 221.0 | 221.0 |
| SD | 243.1 | 246.8 | 183.3 | 4,308.8 | 79.8 | 138.5 | 443.6 | 77.8 | 190.2 |
| 95% CI | 51.9 | 16.5 | 14.7 | 107.3 | 29.8 | 25.3 | 19.6 | 33.9 | 18.5 |
| >1 yr | |||||||||
| Mean | 167.7 | 80.4 | 113.3 | 399.7 | 101.7 | 91.1 | 108.0 | 266.4 | 131.2 |
| Median | 93.2 | 22.9 | 48.0 | 357.2 | 81.5 | 66.2 | 106.5 | 199.0 | 95.0 |
| SD | 184.7 | 224.6 | 129.0 | 188.0 | 70.1 | 83.7 | 49.8 | 262.0 | 130.8 |
| 95% CI | 14.8 | 7 | 35.7 | 60 | 12.1 | 13.1 | 11 | 18.3 | 15.3 |
Assessment 2.
Assessment 1.
CI, confidence interval.
The Elecsys assay detected low or equivocal IgG titers, as confirmed by the reference method, the dye test, in 97.8% of cases (panel 3) (Table 3; see also Table S2 in the supplemental material), while such titers were detected in only 88.9%, 84.4%, 73.3%, and 66.7% of sera with the Enzygnost, Architect, Platelia, and Advia Centaur assays, respectively. The Liaison assay detected low or equivocal titers in only 17.8% of cases. As for the AxSYM and VIDIA assays, 100% of equivocal IgG titers were detected, whereas 87.5% were detected when using the VIDAS assay.
TABLE 3.
IgG detection in nine assays from 15 patients in assessment 1 and 45 patients in assessment 2 with low or equivocal IgG titers without IgM
| Detection | Performance (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Advia Centaura | Architecta | Elecsysa | Enzygnosyta | Liaisona | Plateliaa | AxSYMb | VIDASb | VIDIAb | |
| Positive | 23 | 16 | 43 | 26 | 3 | 28 | 12 | 11 | 6 |
| Equivocal | 7 | 22 | 1 | 14 | 5 | 5 | 3 | 3 | 9 |
| Negative | 15 | 7 | 1 | 5 | 37 | 12 | 0 | 1 | 0 |
Assessment 2.
Assessment 1.
Clinical specificity for IgG antibodies was calculated and expressed as a percentage of IgG false positives found in sera taken from patients with interfering diseases who were negative for anti-toxoplasma IgG antibodies when using the reference methods (panel 2).
The percentages of false positives observed were 0.01% with the VIDIA assay, 4.3% with the VIDAS assay, and 8.6% with the AxSYM assay. No false-positive IgG results were found with the Enzygnost, Platelia, Advia Centaur, Architect, or Liaison assays, whereas 3.6% of false positives were noted with the Elecsys assay. Interferences were observed in sera positive for ANA, rheumatoid factor, syphilis IgM, or CMV, HA, hepatitis B, herpes simplex, and Epstein-Barr viruses.
Performance of IgM detection.
Sensitivities for detecting IgM antibodies based on results from panel 1 varied greatly from one assay to another (Table 4), and the best sensitivity (97.9%) was observed when using the Platelia assay. Four assays displayed a sensitivity between 80% and 90%, namely, the Architect (80.9%), Enzygnost (84.2%), AxSYM (86%), and Advia Centaur (89.4%) assays. As for the VIDIA, Elecsys, and VIDAS assays, their sensitivities were less than 80% (79%, 76.6%, and 65%, respectively).
TABLE 4.
Performance comparison of the nine assays in T. gondii-specific IgM antibody detection on routine panelsa and concordance between positive IgM detection and confirmation by the reference method (ISAGA-M) in chronic (>1 year) toxoplasmosis patientsb
| Assay | Performance (%) |
||||
|---|---|---|---|---|---|
| Sensitivityc | Specificityd | PPV | NPV | Concordance with ISAGA-M | |
| AdviaCentaure | 89.4 | 98.1 | 85.7 | 98.6 | 81 |
| Architecte | 80.9 | 99.7 | 97.4 | 97.6 | 95.2 |
| AxSYMf | 86 | 100 | 100 | 97.9 | 60 |
| Elecsyse | 76.6 | 96.7 | 75 | 97 | 92.9 |
| Enzygnoste | 84.2 | 99.7 | 97 | 98.4 | 16.7 |
| Liaisone | 61.7 | 98.4 | 82.4 | 95.3 | 59.5 |
| Plateliae | 97.9 | 92.6 | 63 | 99.7 | 97.6 |
| VIDASf | 65 | 99.7 | 90.5 | 94.9 | 70 |
| VIDIAf | 79 | 97.9 | 90.5 | 94.3 | 60 |
Panels containing 419 serum samples in assessment 1 and 406 serum samples in assessment 2.
Panels containing 19 serum samples in assessment 1 and 45 serum samples in assessment 2.
Sensitivity = true positive/(true positive + false negative).
Specificity = true negative/(true negative + false positive).
Assessment 2.
Assessment 1.
Eight assays presented a specificity higher than 95%, and the Platelia assay was the least specific (92.6%).
All the NPVs were between 94.3% (VIDIA) and 99.7% (Platelia), whereas the PPVs were highly variable, with extremes of 100% for the AxSYM assay and 63% for the Platelia assay.
Detection of residual IgM antibodies following a minimum of 1 year in chronic toxoplasmosis cases, as confirmed by the reference method (ISAGA-M), was superior or equal to 60% for seven out of the nine assays tested, achieving over 90% with the Platelia, Architect, and Elecsys assays, in 97.6%, 95.2%, and 92.9%, respectively, of sera analyzed. The least-sensitive assay for residual IgM antibodies was the Enzygnost assay, with only 16.7% of positive residual IgM antibodies detected (Table 4).
Clinical specificities for detecting IgM antibodies in potentially interfering viral, bacterial, or autoimmune diseases were 100% for most of the assays analyzed; only one false-positive result was observed (with the AxSYM assay [specificity, 99.9%], in a patient testing positive for HA virus IgM antibodies).
Performance in early IgG detection for acute infection.
Detailed antibody kinetics was not assessed given that the cases were selected a posteriori. Thus, intervals between sampling times differed from one case to another, and each patient's treatment was initiated immediately after the first positive serum test was observed. The data presented here concerned medical records for which discordance for IgM or IgG in the first serum sample (seven records for assessment 1 and 15 records for assessment 2) was reported, whereas IgG seroconversion identified in follow-up tests allowed progressive infection to be confirmed.
For assessment 1 (Table 5; see also Table S3), in eight records (cases A, H, I, J, K, M, N, and O) there were no differences in IgM and IgG appearance kinetics. For the seven records with discordance, IgM antibodies were positively identified in six cases with three assays, and equivocally identified with the VIDIA assay (record G). The observed differences mainly concerned the IgG antibodies that were detected earlier with the AxSYM assay. Detection of these antibodies was positive for records C and D, equivocal with assays VIDIA and VIDAS (record C), and negative for record D when using the same assays. Equivocal IgG levels were detected with the AxSYM assay but were negative with the two other assays for records B and F.
TABLE 5.
Comparison of early IgM and IgG detection confirmed in 15 cases of acute toxoplasmosis with AxSYM, VIDIA, and VIDAS assays in assessment 1a
| Case | AxSYM |
VIDIA |
VIDAS |
|||
|---|---|---|---|---|---|---|
| IgM | IgG | IgM | IgG | IgM | IgG | |
| A | + | + | + | + | + | + |
| B | + | e | + | − | + | − |
| C | + | + | + | e | + | e |
| D | + | + | + | − | + | − |
| E | + | − | + | − | + | e |
| F | + | e | + | − | + | − |
| G | + | + | e | + | + | + |
| H | + | + | + | + | + | + |
| I | + | + | + | + | + | + |
| J | + | + | + | + | + | + |
| K | + | + | + | + | + | + |
| L | + | + | + | − | + | + |
| M | + | + | + | + | + | + |
| N | + | + | + | + | + | + |
| O | + | + | + | + | + | + |
| Total | ||||||
| Positive | 15 | 12 | 14 | 9 | 15 | 10 |
| Equivocal | 0 | 2 | 1 | 1 | 0 | 2 |
| Negative | 0 | 1 | 0 | 5 | 0 | 3 |
e, equivocal result; +, positive result; −, negative result.
For assessment 2 (Table 6; see also Table S4), only one out of 17 records showed identical results with the six assays tested (not analyzed) for the 16 discordant cases. Positive detection of IgM antibodies was obtained in 94% of cases (15/16) with the Elecsys assay, whereas the Enzygnost assay detected only 31% of cases that were positive for IgM (5/16).
TABLE 6.
Comparison of IgM and IgG early detection with six assays confirmed in 16 cases of acute toxoplasmosis in assessment 2a
| Case | Advia Centaur |
Architect |
Elecsys |
Enzygnost |
Liaison |
Platelia |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | IgM | IgG | |
| 1 | + | − | + | − | + | e | + | − | + | − | + | + |
| 2 | + | − | + | e | + | e | − | − | + | − | + | − |
| 3 | + | − | + | − | + | e | − | − | + | − | + | − |
| 4 | − | − | − | e | − | − | − | − | − | − | − | − |
| 5 | − | − | − | − | + | e | − | − | − | − | + | − |
| 6 | + | − | e | − | + | e | − | e | + | − | + | − |
| 7 | + | − | + | − | + | − | − | − | − | − | + | − |
| 8 | + | − | + | + | + | e | + | + | + | e | + | − |
| 9 | + | − | + | e | + | − | − | − | + | − | + | − |
| 10 | + | − | + | e | + | e | + | − | + | − | + | − |
| 11 | + | − | + | − | + | e | + | − | + | − | + | − |
| 12 | + | − | + | − | + | − | − | − | + | − | + | − |
| 13 | + | − | + | e | + | e | − | − | + | − | + | − |
| 14 | + | − | + | − | + | e | − | − | e | − | + | − |
| 15 | − | − | − | − | + | e | − | − | − | − | − | − |
| 16 | − | + | + | + | e | + | e | + | + | + | + | |
| Total | − | |||||||||||
| Positive | 13 | 0 | 11 | 2 | 15 | 0 | 5 | 1 | 11 | 1 | 14 | 2 |
| Equivocal | 0 | 0 | 1 | 5 | 0 | 14 | 0 | 2 | 1 | 1 | 0 | 0 |
| Negative | 3 | 16 | 3 | 9 | 1 | 2 | 11 | 13 | 4 | 14 | 2 | 14 |
e, equivocal result; +, positive result; −, negative result.
As for IgG, we noticed that the Elecsys assay was associated with the quickest detection and with equivocal titers in 75% of cases. Positive (cases 8 and 16) or equivocal titers were also detected in 44% of cases with the Architect assay. Concerning other assays, detection of positive or equivocal titers was below 20% for the Enzygnost, Liaison, and Platelia assays, while IgG detection was negative with the Advia Centaur assay.
DISCUSSION
The diagnosis of T. gondii infection, which is asymptomatic in >80% of cases in Europe and North America, is essentially based on serological tests for IgG and IgM antibodies (34). The requirements for the performance of assays to be used vary depending on the clinical context. On screening (first-intention method), the precocity of IgM appearance and the test's specificity are decisive for diagnosing an initial T. gondii infection (28), whereas the presence of IgG antibodies enables immunization against T. gondii to be considered. The choice of which assay to use should take its whole performance into account in addition to the laboratory's technical and financial constraints (11).
IgM antibodies are the first serological markers to become positive following Toxoplasma infection (35). Another study focused on toxoplasmosis IgA detection is currently ongoing in the network. On the basis of the assays tested, a large variability in specificities from previous studies was reported (13, 31, 33, 36). High specificity should be targeted to ensure the test's reliability in the event of suspected acute toxoplasmosis. Regarding the assays assessed on a routine basis, good specificities were observed with AxSYM, Architect, Enzygnost, and VIDAS, exceeding 99%, with PPVs above 90%. The five other assays tested also showed high specificities ranging from 92.6% to 99.4%, yet with lower PPVs ranging from 63% to 85.7%. Access tests were excluded due to their poor specificity for IgG detection. None of the nine assays tested detected false-positive IgM antibodies in sera originating from patients with potentially interfering diseases.
Whereas the specificities of the assays were excellent, their sensitivities revealed lower performance in the routine panels; the sensitivity of only five of the tested assays exceeded 80% (Platelia, Advia Centaur, AxSYM, Enzygnost, and Architect assays). Of these panels, Platelia had the highest detection sensitivity (97.9%) associated with a 99.7% NPV. Likewise, the Advia Centaur, AxSYM, Enzygnost, and Architect assays showed excellent performance with respect to sensitivity (89.4%, 86%, 84.2%, and 80.9%, respectively), along with high NPVs (98.6%, 97.9%, 98.4%, and 97.6%, respectively). In patients suffering from acute infection, the AxSYM, VIDAS, VIDIA and Elecsys assays displayed detection sensitivities higher than 90% (100% for the three first assays and 94% for Elecsys). As for Enzygnost, IgM antibodies were detected at a later time point, though only in 31.1% of cases. The difference in IgM sensitivities observed between routine and acute toxoplasmosis populations was probably accounted for by the selected antigen specificities of the assays (37, 38).
Detecting residual IgM antibodies is considered common in chronic toxoplasmosis patients (39). A selection of assays with the lowest capacity to detect these residual IgM antibodies would be preferred. Another alternative would be to further develop assays using recombinant antigens that are not recognized by these residual IgM antibodies. Seven out of the nine assays tested revealed residual IgM antibodies exceeding 60%, as confirmed by the reference ISAGA-M method, in chronic toxoplasmosis cases lasting for >1 year. The Enzygnost assay only detected 16.7% of residual IgM antibodies. Given the presence of positive IgM and IgG antibodies in the first serum sample, a second-intention determination, using the same serum, of IgG avidity (when it is high) enabled confirmation of the infection as chronic (12, 40–42).
Concerning Toxoplasma IgG antibodies, low and stable specific IgG antibody titers, in the absence of associated IgM antibodies, are indicative of an older infection. Given this situation, no serological follow-up during pregnancy is required, whereas such a scenario allows estimating the reactivation risk in immunosuppressed patients. In the absence of IgG antibodies, appropriate prophylactic measures should be implemented in patient populations at risk. Every reagent assay tested on routine sera revealed IgG sensitivity values exceeding 99.5%. Yet, in our case, we observed that IgG titers varied by a factor 2 to 10 depending on the reagent assay, with the Elecsys assay showing the highest titers. Such a discrepancy between IgG titers expressed in international units (IU/ml) (43) for the same serum has been reported (44). This could, at least to some extent, be accounted for by the selected antigenic combinations (37, 45, 46) and the use of standards from different generations (World Health Organization [WHO]). If IgG titer follow-up proves necessary, it is recommended that a follow-up be conducted by the same laboratory with the same method (17). In the presence of a very low specific IgG antibody titer, close to the detection limit (equivocal IgG), this can lead us to consider the absence of prior immunization while implementing an iterative, expansive, and unjustified follow-up, notably in the event of pregnancy. The positivity threshold (cutoff) for IgG antibodies differs from one assay to another (47). It is recommended to carry out a second-intention method using the same serum to confirm the results (48, 49) or use a reference laboratory before drawing any final conclusion regarding the patient's immune status in the absence of any immunosuppression. Among these second-intention methods, the historical favorite is the dye test. However, due to its scarce availability in reference laboratories, an immunoblotting procedure was developed. Its performance proved to be similar to that of the dye test, thereby allowing us to commonly use this assay (47).
As for sera with a low or equivocal IgG antibody titer, in the absence of IgM antibodies, the AxSYM, VIDIA, and Elecsys assays displayed the highest sensitivities (100%, 100%, and 97.8%, respectively), in line with previous study data (25, 49). On the contrary, the Liaison assay had the poorest detection threshold, with only 17.8% of low or equivocal IgG titers detected.
In several clinical settings, such as autoimmune, infectious, or other diseases, as well as in immunosuppression cases, false-positive IgG antibody titers may occur (50) and possibly lead to wrong conclusions with respect to the patient's immunization status and serological follow-up requirements. In such cases, the real absence of immunization exposes patients to the risk of acute toxoplasmosis, as no serological follow-up is being conducted. Determination of specific IgG antibody titers via two different methods decreases this risk. In our assessment, clinical specificity of most reagent assays exceeded 99% in patients with potentially interfering diseases, in the absence of any immunosuppression context, with only the VIDAS and AxSYM assays exhibiting lower performance (95.7% and 91.4%, respectively).
The precocity of IgG detection is a key element in cases with isolated positive IgM antibody titers. When such a scenario is observed in pregnant women, this leads us to consider the possibility of a recent infection, rendering it essential to confirm this infection before implementing a treatment designed to decrease fetal transmission and involvement (51, 52). The AxSYM and Elecsys IgG assays were found to be the most efficient methods in the patients in whom a seroconversion was assessed (Tables 5 and 6). The IgG antibody titers were positive or equivocal in 85.6 and 75% of cases, respectively, while IgG titers turned positive at a later time point when using the other assays. Treatment initiation might account for the observed differences in kinetics. The discovery of low or equivocal IgG titers by commercialized assays in the presence of IgM antibodies should encourage us to utilize a Western blot LD BIO-Toxo II method, which has been proven to be more sensitive and highly specific and might be very useful for confirming T. gondii infection (47, 53).
In conclusion, the criteria for assay selection should take into account the assay's specificity and sensitivity for IgM detection, which are determinants for diagnosing recent Toxoplasma infection. The first-line selection criteria for IgG detection consist of maximum specificity, along with detection sensitivity for low or equivocal titers in both chronic and recent toxoplasmosis infection cases. In more complex situations, specialized reference centers should be able to solve these enigmatic cases by using nonautomated second-line reference methods.
The AxSYM, VIDAS, Architect, and Elecsys assays displayed excellent performance for IgG and IgM detection. Whereas IgM detection performance of the Platelia assay was similar, its capability for early IgG antibody detection was lower in both routine settings and progressive toxoplasmosis cases.
The Enzygnost assay exhibited low IgM antibody sensitivity, along with delayed IgG antibody detection sensitivity in recent Toxoplasma infections. The Liaison and Advia Centaur assays also exhibited minor performance in detecting low IgG antibody titers. Lack of sensitivity in IgG detection can be explained by the selected reagent cutoff thresholds, along with the antigen types used.
Recent studies revealed that choosing recombinant chimeric multiepitope antigens (54, 55) was instrumental in improving the performance of commercialized enzyme immunoassay kits in detecting T. gondii-specific antibodies, using either soluble antigens from crude extracts or recombinant antigens (56–58). The selection of the reagent that allows for both IgG and IgM detection should take into account the assay's performance under both routine conditions and specified clinical settings (such as high sensitivity for IgM in pregnancy and for IgG in immunosuppressed patients). A good understanding of a particular assay system's inherent weaknesses may be associated with the implementation of second-intention methods that allow the results to be confirmed, such as avidity or Western blot for IgG antibodies and ISAGA-M for IgM antibodies.
Supplementary Material
ACKNOWLEDGMENTS
This study was initiated by the Serology Group of the French National Reference Center for Toxoplasmosis (Centre National de Référence de la Toxoplasmose).
This study was funded by the Department of Infectious Diseases, French Institute for Public Health Surveillance (InVS), Saint Maurice, France.
The members of the National Reference Center for Toxoplasmosis are as follows: A. Totet and C. Damaini (Hospital and University Centre Amiens); B. Cimon (Hospital and University Centre Angers); E. Scherrer and F. Grenouillet (Hospital and University Centre Besançon); I. Accoceberry and F. Gabriel (Hospital and University Centre Bordeaux); G. Nevez, D. Quinio, and E. Moalic (Hospital and University Centre Brest); J. Bonhomme (Hospital and University Centre Caen); M. Demar (Hospital and University Centre Cayenne); F. Botterel-Chartier and F. Foulet (Hospital and University Centre Créteil); B. Cuisenier, F. Dalle, and S. Valot (Hospital and University Centre Dijon); M. P. Brenier-Pinchart, H. Fricker-Hidalgo, and H. Pelloux (Hospital and University Centre Grenoble); M. Nicolas (Hospital and University Centre Guadeloupe); B. Sendid, A. S. Delplancque, and E. Frealle (Hospital and University Centre Lille); D. Ajzenberg, J. B. Murat, and M. L. Dardé (Hospital and University Centre Limoges); C. L'Ollivier and R. Piarroux (Hospital and University Centre Marseille); N. Desbois (Hospital and University Centre Martinique); P. Bastien, Y. Sterkers, S. Albaba, and L. Lachaud (Hospital and University Centre Montpellier); M. Machouart and A. Debourgogne (Hospital and University Centre Nancy); R. A. Lavergne and F. Morio (Hospital and University Centre Nantes); N. Ferret, C. Pomares, and P. Marty (Hospital and University Centre Nice); S. Houze (Hospital and University Centre Paris Bichat); A. Angoulvant and N. Dahane (Hospital and University Centre Paris Bicêtre); H. Yera (Hospital and University Centre Paris Cochin); J. Menotti and N. Guigue (Hospital and University Centre Paris St. Louis); F. Touafek and L. Paris (Hospital and University Centre Paris Salpetrière); N. Godineau (Hospital and University Centre Paris St Denis); M. E. Bougnoux (Hospital and University Centre Paris Necker); C. Hennequin and G. Belkadi (Hospital and University Centre Paris Saint-Antoine); E. Perraud and M. H. Rodier (Hospital and University Centre Poitiers); D. Aubert, C. Chemla, F. Foudrinier, and I. Villena (Hospital and University Centre Reims); F. Robert-Gangneux (Hospital and University Centre Rennes); L. Favennec and G. Gargala (Hospital and University Centre Rouen); P. Flori and R. Raberin (Hospital and University Centre St Etienne); D. Filisetti and O. Villard (Hospital and University Centre Strasbourg); J. Fillaux and S. Cassaing (Hospital and University Centre Toulouse); and N. Vanlangendonck (Hospital and University Centre Tours).
The authors declare that they have no conflicts of interest with regard to this study.
Funding Statement
The study was funded by a recurrent grant (2012 to 2016) from Institut National de Veille Sanitaire (INVS).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01193-16.
REFERENCES
- 1.Remington JS, Thulliez P, Montoya JG. 2004. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol 42:941–945. doi: 10.1128/JCM.42.3.941-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer KM, Holfels E, Roizen N, Swisher C, Mack D, Remington J, Withers S, Meier P, McLeod R, Toxoplasmosis Study Group. 2005. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: implications for prenatal management and screening. Am J Obstet Gynecol 192:564–571. doi: 10.1016/j.ajog.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Nissapatorn V, Noor Azmi MA, Cho SM, Fong MY, Init I, Rohela M, Khairul Anuar A, Quek KF, Latt HM. 2003. Toxoplasmosis: prevalence and risk factors. J Obstet Gynaecol 23:618–624. doi: 10.1080/01443610310001604376. [DOI] [PubMed] [Google Scholar]
- 4.Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. 2001. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol 154:357–365. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- 5.Fuente MC, Bovone NS, Cabral GE. 1997. Prophylaxis of prenatal toxoplasmosis. Medicina (B Aires) 57:155–160. (In Spanish.) [PubMed] [Google Scholar]
- 6.Villena I, Ancelle T, Delmas C, Garcia P, Brezin AP, Thulliez P, Wallon M, King L, Goulet V, Toxosurv network and National Reference Centre for Toxiplasmosis. 2010. Congenital toxoplasmosis in France in 2007: first results from a national surveillance system. Euro Surveill 15:pii:19600 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19600. [DOI] [PubMed] [Google Scholar]
- 7.Nogareda F, Le Strat Y, Villena I, De Valk H, Goulet V. 2014. Incidence and prevalence of Toxoplasma gondii infection in women in France, 1980–2020: model-based estimation. Epidemiol Infect 142:1661–1670. doi: 10.1017/S0950268813002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montoya JG, Remington JS. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 47:554–566. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 9.Mele A, Paterson PJ, Prentice HG, Leoni P, Kibbler CC. 2002. Toxoplasmosis in bone marrow transplantation: a report of two cases and systematic review of the literature. Bone Marrow Transplant 29:691–698. doi: 10.1038/sj.bmt.1703425. [DOI] [PubMed] [Google Scholar]
- 10.Pfaff AW, de-la-Torre A, Rochet E, Brunet J, Sabou M, Sauer A, Bourcier T, Gomez-Marin JE, Candolfi E. 2014. New clinical and experimental insights into Old World and neotropical ocular toxoplasmosis. Int J Parasitol 44:99–107. doi: 10.1016/j.ijpara.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Villard O, Cimon B, Franck J, Fricker-Hidalgo H, Godineau N, Houze S, Paris L, Pelloux H, Villena I, Candolfi E, Network from the French National Reference Center for Toxplasmosis. 2012. Evaluation of the usefulness of six commercial agglutination assays for serologic diagnosis of toxoplasmosis. Diagn Microbiol Infect Dis 73:231–235. doi: 10.1016/j.diagmicrobio.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Villard O, Breit L, Cimon B, Franck J, Fricker-Hidalgo H, Godineau N, Houze S, Paris L, Pelloux H, Villena I, Candolfi E, French National Reference Center for Toxoplasmosis Network 2013. Comparison of four commercially available avidity tests for Toxoplasma gondii-specific IgG antibodies. Clin Vaccine Immunol 20:197–204. doi: 10.1128/CVI.00356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liesenfeld O, Press C, Montoya JG, Gill R, Isaac-Renton JL, Hedman K, Remington JS. 1997. False-positive results in immunoglobulin M (IgM) toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J Clin Microbiol 35:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecolier B, Pucheu B. 1993. Value of the study of IgG avidity for the diagnosis of toxoplasmosis. Pathol Biol (Paris) 41:155–158. (In French.) [PubMed] [Google Scholar]
- 15.Lachaud L, Calas O, Picot MC, Albaba S, Bourgeois N, Pratlong F. 2009. Value of 2 IgG avidity commercial tests used alone or in association to date toxoplasmosis contamination. Diagn Microbiol Infect Dis 64:267–274. doi: 10.1016/j.diagmicrobio.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Candolfi E, Pastor R, Huber R, Filisetti D, Villard O. 2007. IgG avidity assay firms up the diagnosis of acute toxoplasmosis on the first serum sample in immunocompetent pregnant women. Diagn Microbiol Infect Dis 58:83–88. doi: 10.1016/j.diagmicrobio.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Villard O, Cimon B, L'Ollivier C, Fricker-Hidalgo H, Godineau N, Houze S, Paris L, Pelloux H, Villena I, Candolfi E. 2016. Serological diagnosis of Toxoplasma gondii infection: recommendations from the French National Reference Center for Toxoplasmosis. Diagn Microbiol Infect Dis 84:22–33. doi: 10.1016/j.diagmicrobio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Sensini A. 2006. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin Microbiol Infect 12:504–512. doi: 10.1111/j.1469-0691.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 19.Walton BC, Benchoff BM, Brooks WH. 1966. Comparison of the indirect fluorescent antibody test and methylene blue dye test for detection of antibodies to Toxoplasma gondii. Am J Trop Med Hyg 15:149–152. [DOI] [PubMed] [Google Scholar]
- 20.Sabin AB, Feldman HA. 1948. Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoon parasite (Toxoplasma). Science 108:660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- 21.Desmonts G. 1963. The role of the laboratory in the diagnosis of toxoplasmosis. Rev Pathol Gen Physiol Clin 63:949–958. (In French.) [PubMed] [Google Scholar]
- 22.Reiter-Owona I, Petersen E, Joynson D, Aspock H, Darde ML, Disko R, Dreazen O, Dumon H, Grillo R, Gross U, Hayde M, Holliman R, Ho-Yen DO, Janitschke K, Jenum PA, Naser K, Olszewski M, Thulliez P, Seitz HM. 1999. The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull World Health Organ 77:929–935. [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy KT, Wharton PJ, Johnson JD, New L, Holliman RE. 1989. Assessment of immunoglobulin-M immunosorbent agglutination assay (ISAGA) for detecting toxoplasma specific IgM. J Clin Pathol 42:1291–1295. doi: 10.1136/jcp.42.12.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cozon GJ, Ferrandiz J, Nebhi H, Wallon M, Peyron F. 1998. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur J Clin Microbiol Infect Dis 17:32–36. doi: 10.1007/BF01584360. [DOI] [PubMed] [Google Scholar]
- 25.Cimon B, Marty P, Morin O, Bessieres MH, Marx-Chemla C, Gay-Andrieu F, Thulliez P. 1998. Specificity of low anti-Toxoplasma IgG titers with IMx and AxSYM Toxo IgG assays. Diagn Microbiol Infect Dis 32:65–67. doi: 10.1016/S0732-8893(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 26.Diepersloot RJ, Dunnewold-Hoekstra H, Kruit-Den Hollander J, Vlaspolder F. 2001. Antenatal screening for hepatitis B and antibodies to Toxoplasma gondii and rubella virus: evaluation of two commercial immunoassay systems. Clin Diagn Lab Immunol 8:785–787. doi: 10.1128/CDLI.8.4.785-787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefer LE, Dyke JW, Meglio FD, Murray PR, Crafts W, Niles AC. 1989. Evaluation of microparticle enzyme immunoassays for immunoglobulins G and M to rubella virus and Toxoplasma gondii on the Abbott IMx automated analyzer. J Clin Microbiol 27:2410–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gay-Andrieu F, Fricker-Hidalgo H, Sickinger E, Espern A, Brenier-Pinchart MP, Braun HB, Pelloux H. 2009. Comparative evaluation of the ARCHITECT Toxo IgG, IgM, and IgG Avidity assays for anti-Toxoplasma antibodies detection in pregnant women sera. Diagn Microbiol Infect Dis 65:279–287. doi: 10.1016/j.diagmicrobio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Okrongly D. 2004. The ADVIA Centaur immunoassay system–designed for infectious disease testing. J Clin Virol 30 Suppl 1:S19–22. doi: 10.1016/j.jcv.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Petersen E, Borobio MV, Guy E, Liesenfeld O, Meroni V, Naessens A, Spranzi E, Thulliez P. 2005. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J Clin Microbiol 43:1570–1574. doi: 10.1128/JCM.43.4.1570-1574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prusa AR, Hayde M, Unterasinger L, Pollak A, Herkner KR, Kasper DC. 2010. Evaluation of the Roche Elecsys Toxo IgG and IgM electrochemiluminescence immunoassay for the detection of gestational Toxoplasma infection. Diagn Microbiol Infect Dis 68:352–357. doi: 10.1016/j.diagmicrobio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Decoster A, Lecolier B. 1996. Bicentric evaluation of Access Toxo immunoglobulin M (IgM) and IgG assays and IMx toxo IgM and IgG assays and comparison with Platelia Toxo IgM and IgG assays. J Clin Microbiol 34:1606–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calderaro A, Peruzzi S, Piccolo G, Gorrini C, Montecchini S, Rossi S, Chezzi C, Dettori G. 2009. Laboratory diagnosis of Toxoplasma gondii infection. Int J Med Sci 6:135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts A, Hedman K, Luyasu V, Zufferey J, Bessieres MH, Blatz RM, Candolfi E, Decoster A, Enders G, Gross U, Guy E, Hayde M, Ho-Yen D, Johnson J, Lecolier B, Naessens A, Pelloux H, Thulliez P, Petersen E. 2001. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur J Clin Microbiol Infect Dis 20:467–474. doi: 10.1007/PL00011289. [DOI] [PubMed] [Google Scholar]
- 35.Naot Y, Remington JS. 1980. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J Infect Dis 142:757–766. doi: 10.1093/infdis/142.5.757. [DOI] [PubMed] [Google Scholar]
- 36.Sickinger E, Gay-Andrieu F, Jonas G, Schultess J, Stieler M, Smith D, Hausmann M, Stricker R, Stricker R, Dhein J, Braun HB. 2008. Performance characteristics of the new ARCHITECT Toxo IgG and Toxo IgG Avidity assays. Diagn Microbiol Infect Dis 62:235–244. doi: 10.1016/j.diagmicrobio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Aubert D, Maine GT, Villena I, Hunt JC, Howard L, Sheu M, Brojanac S, Chovan LE, Nowlan SF, Pinon JM. 2000. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol 38:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drapala D, Holec-Gasior L, Kur J, Ferra B, Hiszczynska-Sawicka E, Lautenbach D. 2014. A new human IgG avidity test, using mixtures of recombinant antigens (rROP1, rSAG2, rGRA6), for the diagnosis of difficult-to-identify phases of toxoplasmosis. Diagn Microbiol Infect Dis 79:342–346. doi: 10.1016/j.diagmicrobio.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Giraldo M, Portela RW, Snege M, Leser PG, Camargo ME, Mineo JR, Gazzinelli RT. 2002. Immunoglobulin M (IgM)-glycoinositolphospholipid enzyme-linked immunosorbent assay: an immunoenzymatic assay for discrimination between patients with acute toxoplasmosis and those with persistent parasite-specific IgM antibodies. J Clin Microbiol 40:1400–1405. doi: 10.1128/JCM.40.4.1400-1405.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelloux H, Bessieres MH, Chemla C, Cimon B, Gay-Andrieu F, Marty P, Rabodonirina M, Thulliez P. 2006. Detection of anti-toxoplasma IgM in pregnant women. Ann Biol Clin (Paris) 64:95 (In French.) [PubMed] [Google Scholar]
- 41.Fricker-Hidalgo H, Saddoux C, Suchel-Jambon AS, Romand S, Foussadier A, Pelloux H, Thulliez P. 2006. New VIDAS assay for Toxoplasma-specific IgG avidity: evaluation on 603 sera. Diagn Microbiol Infect Dis 56:167–172. doi: 10.1016/j.diagmicrobio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Sickinger E, Braun HB, Praast G, Stieler M, Gundlach C, Birkenbach C, Prostko J, Palafox MA, Frias E, Hsu S, Matias M, Pucci D, Hausmann M, Sagel U, Smith D. 2009. Evaluation of the Abbott Architect Toxo IgM assay. Diagn Microbiol Infect Dis 64:275–282. doi: 10.1016/j.diagmicrobio.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Rigsby P, Rijpkema S, Guy EC, Francis J, Das RG. 2004. Evaluation of a candidate international standard preparation for human anti-Toxoplasma immunoglobulin G. J Clin Microbiol 42:5133–5138. doi: 10.1128/JCM.42.11.5133-5138.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maudry A, Chene G, Chatelain R, Patural H, Bellete B, Tisseur B, Hafid J, Raberin H, Beretta S, Sung RT, Belot G, Flori P. 2009. Bicentric evaluation of six anti-Toxoplasma immunoglobulin G (IgG) automated immunoassays and comparison to the Toxo II IgG Western blot. Clin Vaccine Immunol 16:1322–1326. doi: 10.1128/CVI.00128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petithory JC, Ambroise-Thomas P, De Loye J, Pelloux H, Goullier-Fleuret A, Milgram M, Buffard C, Garin JP. 1996. Serodiagnosis of toxoplasmosis: a comparative multicenter study of a standard scale through various actual tests and expression of the results in international units. Groupe de travail toxoplasmose du Contrôle national de qualité en parasitologie. Syndicat des fabricants de réactifs de laboratoire. Groupe de travail standardisation des tests sérologiques du Réseau européen de lutte contre la toxoplasmose congénitale. Bull World Health Organ 74:291–298. (In French.) [PMC free article] [PubMed] [Google Scholar]
- 46.Pietkiewicz H, Hiszczynska-Sawicka E, Kur J, Petersen E, Nielsen HV, Stankiewicz M, Andrzejewska I, Myjak P. 2004. Usefulness of Toxoplasma gondii-specific recombinant antigens in serodiagnosis of human toxoplasmosis. J Clin Microbiol 42:1779–1781. doi: 10.1128/JCM.42.4.1779-1781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franck J, Garin YJ, Dumon H. 2008. LDBio-Toxo II immunoglobulin G Western blot confirmatory test for anti-toxoplasma antibody detection. J Clin Microbiol 46:2334–2338. doi: 10.1128/JCM.00182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flori P, Chene G, Varlet MN, Sung RT. 2009. Toxoplasma gondii serology in pregnant woman: characteristics and pitfalls. Ann Biol Clin (Paris) 67:125–133. (In French.) [DOI] [PubMed] [Google Scholar]
- 49.Lesle F, Touafek F, Fekkar A, Mazier D, Paris L. 2011. Discrepancies between a new highly sensitive Toxoplasma gondii ELISA assay and other reagents: interest of Toxo IgG Western blot. Eur J Clin Microbiol Infect Dis 30:1207–1212. doi: 10.1007/s10096-011-1214-1. [DOI] [PubMed] [Google Scholar]
- 50.Roux-Buisson N, Fricker-Hidalgo H, Foussadier A, Rolland D, Suchel-Jambon AS, Brenier-Pinchart MP, Pelloux H. 2005. Comparative analysis of the VIDAS Toxo IgG IV assay in the detection of antibodies to Toxoplasma gondii. Diagn Microbiol Infect Dis 53:79–81. doi: 10.1016/j.diagmicrobio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Wallon M, Liou C, Garner P, Peyron F. 1999. Congenital toxoplasmosis: systematic review of evidence of efficacy of treatment in pregnancy. BMJ 318:1511–1514. doi: 10.1136/bmj.318.7197.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lefevre-Pettazzoni M, Bissery A, Wallon M, Cozon G, Peyron F, Rabilloud M. 2007. Impact of spiramycin treatment and gestational age on maturation of Toxoplasma gondii immunoglobulin G avidity in pregnant women. Clin Vaccine Immunol 14:239–243. doi: 10.1128/CVI.00311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jost C, Touafek F, Fekkar A, Courtin R, Ribeiro M, Mazier D, Paris L. 2011. Utility of immunoblotting for early diagnosis of toxoplasmosis seroconversion in pregnant women. Clin Vaccine Immunol 18:1908–1912. doi: 10.1128/CVI.05303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai JF, Jiang M, Qu LL, Sun L, Wang YY, Gong LL, Gong RJ, Si J. 2013. Toxoplasma gondii: enzyme-linked immunosorbent assay based on a recombinant multiepitope peptide for distinguishing recent from past infection in human sera. Exp Parasitol 133:95–100. doi: 10.1016/j.exppara.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Ferra B, Holec-Gasior L, Kur J. 2015. A new Toxoplasma gondii chimeric antigen containing fragments of SAG2, GRA1, and ROP1 proteins-impact of immunodominant sequences size on its diagnostic usefulness. Parasitol Res 114:3291–3299. doi: 10.1007/s00436-015-4552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lecordier L, Fourmaux MP, Mercier C, Dehecq E, Masy E, Cesbron-Delauw MF. 2000. Enzyme-linked immunosorbent assays using the recombinant dense granule antigens GRA6 and GRA1 of Toxoplasma gondii for detection of immunoglobulin G antibodies. Clin Diagn Lab Immunol 7:607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z, Yan C, Liu P, Yan R, Feng Z. 2009. Prevalence of serum antibodies to TORCH among women before pregnancy or in the early period of pregnancy in Beijing. Clin Chim Acta 403:212–215. doi: 10.1016/j.cca.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 58.Liesenfeld O, Press C, Flanders R, Ramirez R, Remington JS. 1996. Study of Abbott Toxo IMx system for detection of immunoglobulin G and immunoglobulin M toxoplasma antibodies: value of confirmatory testing for diagnosis of acute toxoplasmosis. J Clin Microbiol 34:2526–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.