Abstract
Background
Transcatheter left atrial appendage closure is an alternative therapy for stroke prevention in atrial fibrillation patients. These procedures are currently guided with transesophageal echocardiography and fluoroscopy in most centers. As intracardiac echocardiography (ICE) is commonly used in other catheter‐based procedures, we sought to determine the safety and effectiveness of intracardiac echocardiography–guided left atrial appendage closure with the Watchman device.
Methods and Results
A total of 27 patients (11 males, 77.0±8.5 years) with atrial fibrillation receiving Watchman left atrial appendage closure under intracardiac echocardiography guidance at a single center were investigated. All patients were implanted successfully. There were no major procedural complications. The overall procedure‐related complication rate was 14.8%, mainly due to access site hematoma. Transesophageal echocardiography demonstrated successful closure of the left atrial appendage in all patients at 45 days after device implant.
Conclusions
Transcatheter left atrial appendage closure with intracardiac echocardiography guidance is safe and feasible.
Keywords: intracardiac echocardiography, left atrial appendage closure, stroke prevention
Subject Categories: Thrombosis, Ischemic Stroke, Imaging, Atrial Fibrillation
Introduction
Atrial fibrillation (AF) is a common arrhythmia worldwide and is associated with a markedly increased risk of stroke.1, 2 Stroke prevention is an important consideration in the treatment of AF. Left atrial appendage (LAA) closure has emerged as an alternative to chronic oral anticoagulant (OAC) use for stroke prevention. PROTECT AF and subsequent trials have demonstrated the efficacy and safety of this approach.3, 4 The main limitation of transcatheter LAA closure has been the procedural risk. Currently, most centers use fluoroscopy and transesophageal echocardiography (TEE) to guide LAA closure.
Intracardiac echocardiography (ICE) imaging was first used in the 1960s. It is now commonly used to guide many transcatheter intervention procedures, including atrial and ventricular septal defect closures and catheter ablation for various arrhythmias.5, 6, 7 The efficacy and safety of ICE‐guided LAA closure using the Watchman device have yet to be rigorously investigated. We sought to evaluate procedure outcomes of ICE‐guided LAA closure with the Watchman device.
Methods
Written informed consent was obtained from each patient. Procedures were performed in accordance with the ethical standards of Homolka Hospital and with the Helsinki Declaration of 1975, revised in 1983. From November 2013 to June 2014, percutaneous LAA closure using the Watchman device was performed with ICE guidance in a total of 27 patients. All patients underwent LAA closure under local anesthesia.
Study Design and Inclusion/Exclusion Criteria
This was an open‐label nonrandomized single‐center study designed to investigate the safety and feasibility of using ICE to guide percutaneous LAA closure. Patients over 18 years old with documented chronic or paroxysmal nonvalvular AF and an estimated life expectancy of at least 2 years with a relative contraindication to OACs such as bleeding history, renal dysfunction, liver dysfunction, high risk of falling, or drug noncompliance were included. Patients had a significantly elevated risk for stroke according to the CHA2DS2‐VASC score and were indicated for OAC therapy according to the current guidelines.8 Exclusion criteria were symptomatic valvular disease, symptomatic carotid disease, pregnancy, or another indication for chronic OAC therapy. Patients were also excluded if there was evidence of intracardiac thrombus on TEE or transthoracic echocardiography.
Intracardiac Echocardiography
ICE images were obtained using an ACUSON AcuNav ultrasound catheter (Siemens Medical Solutions, USA, Inc., Mountain View, CA). The 10‐Fr catheter was used along with the VIVIDi imaging system (GE Healthcare, Fairfield, CT). ICE guidance was used to confirm the transseptal puncture, to estimate LAA size for the device selection, to verify the delivery sheath position, to confirm the device location and stability, and to detect procedural complications.
Device Implantation
A TEE was performed 1 to 7 days before the procedure to rule out the presence of intracardiac thrombus and to assess the dimensions and the morphology of the LAA. The LAA closure procedure was performed under ICE guidance in a catheterization laboratory with diagnostic imaging equipment. After cannulation of the femoral veins bilaterally, a standard transseptal access system and a 0.035‐inch guidewire with exchange length/extra support (eg, Amplatz Super stiff 260 cm) was introduced via the right femoral vein. An AcuNav ICE catheter was inserted via the left femoral vein and positioned in the right atrium. Location of the transseptal puncture was selected and confirmed by ICE. After transseptal puncture, a guidewire was advanced into the left upper pulmonary vein and the transseptal access system was removed. A single curved access sheath over 5‐Fr pigtail catheter was advanced into the left atrium, and selective LAA angiography was performed with the pigtail catheter (Figure). Prior to device delivery, the pigtail catheter was advanced into the distal portion of the LAA. Subsequently the access sheath was advanced to the distal aspect of the LAA. The marker bands (21, 27, and 33 mm) on the Watchman access sheath served as a guide for appropriate device selection and positioning. After removal of the pigtail catheter, an appropriately sized device based on ICE, LAA angiography, previous TEE measurements, and operator experience was slowly advanced in the access sheath. The device was deployed and/or repositioned until an optimal position was achieved with retraction of the access sheath. After confirming the absence of substantial residual leak by angiography and ICE, attachment of the device was checked with final tug test (a mechanical pulling test). The device was released after final confirmation by both ICE and angiography. Patients were hospitalized overnight and discharged the next day after exclusion of pericardial effusion by transthoracic echocardiography. At 45 days after implantation, a TEE was performed to assess for closure of the LAA as well as the absence of thrombus on the face of the device.
Figure 1.
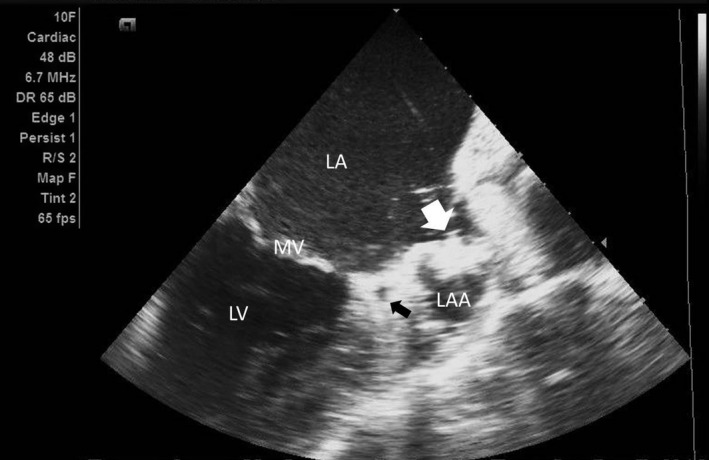
This figure demonstrates the ICE view of the left atrium (LA) and left atrial appendage (LAA) as seen from the right atrium. The long axis of the LAA, the left circumflex artery (black arrow), the mitral valve (MV), and the Watchman device (white arrow) are shown. ICE indicates intracardiac echocardiography; LV indicates left ventricle.
Medical Treatment
For local anesthesia, a total of 20 mL of 1% lidocaine was injected bilaterally at the femoral access sites. After groin access, heparin was given to keep activated clotting time above 250 s. Patients remained on warfarin (PT‐INR 2–3) for a minimum of 45 days after device implantation. Patients with a significant contraindication to warfarin were treated with one of the following:
Non–vitamin K antagonist oral anticoagulation daily.
100 mg aspirin plus 75 mg clopidogrel daily.
Low‐molecular‐weight heparin s.c. 0.01 mg/kg bid for 45 days, followed by 100 mg aspirin plus 75 mg clopidogrel daily for another 4.5 months, finally followed by 100 mg aspirin lifelong.
If echocardiographic criteria for successful LAA closure were fulfilled at 45‐day follow‐up,9 OACs or low‐molecular‐weight heparin were discontinued. Aspirin and clopidogrel were continued for an additional 6 months, followed by aspirin alone indefinitely thereafter.
End Points
The primary end point of the study was successful Watchman implantation. The primary safety end point was occurrence of procedure‐related complications. Other end points were sealing of the LAA and the occurrence of adverse events within 45 days. Successful sealing of the LAA was determined by TEE for the absence of flow or minimal flow (jet of <5‐mm width) around the device according to echocardiographic sealing criteria described previously.9 The sealing was confirmed at 2 different time points: at the end of the implantation procedure and 45 days after implant. Major adverse events were defined as death, stroke including transient ischemic attack, systemic embolism, and major bleeding requiring invasive treatment or blood transfusion.
Statistical Analysis
Estimated frequencies of event occurrence are expressed as percentages or rates. Continuous variables are summarized by mean and SD.
Results
Baseline Characteristics
Patients (n=27; age 77.0±8.5 years; male/female: 11/16) were enrolled in the analysis. The mean CHADS2 score was 3.3±1.4, the mean CHA2DS2VASC score was 5.3±1.6, and the mean HASBLED score was 4.4±1.1 (Table 1). All patients had successful implantation; procedure findings are shown in Table 2. Total fluoroscopy time was 2.9±0.8 minutes. A mean of 1.1±0.3 (range 1–2) devices per patient were used per patient. The mean length of hospitalization was 3.1±1.8 days.
Table 1.
Baseline Patient Characteristics
| n=27 | |
|---|---|
| Age, y | 77.0±8.5 |
| Sex (male/female) | 11 (40.7%)/16 (59.3%) |
| Type of AF | |
| Chronic | 14 (51.9%) |
| Persistent | 9 (33.3%) |
| Paroxysmal | 4 (14.8%) |
| Mean CHADS2 score | 3.3±1.4 |
| CHADS2 score (n) | |
| 0 to 1 | 1 |
| 2 | 8 |
| 3 | 9 |
| 4 | 3 |
| 5 | 3 |
| 6 | 3 |
| Mean CHA2DS2VASc score | 5.3±1.6 |
| CHA2DS2VASc score (n) | |
| 0 to 1 | 0 |
| 2 | 3 |
| 3 | 6 |
| 4 | 7 |
| 5 | 5 |
| 6 | 3 |
| 7 | 2 |
| 8 | 1 |
| 9 | 1 |
| Congestive heart failure or LVEF <40% | 15 (55.6%) |
| Hypertension | 24 (88.9%) |
| Age ≥75 y | 18 (66.7%) |
| Age ≥65 y | 25 (92.6%) |
| Diabetes mellitus | 12 (44.4%) |
| Prior stroke or transient ischemic attack | 10 (37.0%) |
| Vascular disease | 14 (51.9%) |
| HASBLED score | 4.4±1.1 |
Values are mean±SD or number (%). AF indicates atrial fibrillation; LVEF, left ventricular ejection fraction.
Table 2.
Procedure Findings
| n=27 | |
|---|---|
| TEE assessment | |
| LAA ostium width, mm | 19.6±3.6 |
| LAA work length, mm | 20.4±5.4 |
| ICE assessment | |
| LAA ostium width, mm | 21.8±2.7 |
| LAA work length, mm | 23.7±3.8 |
| ICE−TEE (⊿) | |
| LAA ostium width, mm | 0.3±4.8 |
| LAA work length, mm | −0.6±4.6 |
| Fluoroscopy time | 2.98±0.8 |
| Dose area product, Gy cm2 | 16±5.6 |
| Number of devices, n | 1.1±0.3 |
Values are mean±SD or number (%). ICE indicates intracardiac echocardiography; LAA, left atrial appendage; TEE, transesophageal echocardiography.
Primary End Point
The primary efficacy end point of successful implantation was met by 100% of patients. There were no major adverse events (Table 3). A pericardial effusion of 4 mm that did not require drainage was seen in 1 patient (3.7%). Puncture site hematoma occurred in 3 patients (11.1%). One patient with a hematoma required surgical repair and prolonged hospitalization; the others resolved with manual compression and did not prolong the hospitalization. One patient had transient dizziness after the procedure, though a magnetic resonance imaging scan of the brain revealed no significant abnormality. The most likely cause was determined to be dehydration from fasting. The overall rate of procedure‐related complications was 14.8%. No major complications were noted.
Table 3.
Perioperative Complications
| n=27 | |
|---|---|
| Major complications | 0 |
| Cardiac tamponade | 0 |
| Stroke/transient ischemic attack | 0 |
| Myocardial infarction | 0 |
| Device dislocation | 0 |
| Other disability | 0 |
| Death | 0 |
| Minor complications | 4 (14.8%) |
| Pericardial effusion | 0 |
| Air embolization | 0 |
| Thrombus | 0 |
| Hematoma | 3 (11.1%) |
| Others | 1 (3.7%) |
| All complications | 4 (14.8%) |
Secondary End Point
At 45‐day follow‐up, all patients showed successful sealing of LAA with no flow (14/27; 51.9%) or minimal residual flow (<5 mm) around the device. All devices remained stable at the site of deployment. Left atrial size and the degree of mitral regurgitation showed no significant change from baseline. There were no occurrences of stroke, transient ischemic attack, death, or pericardial bleeding complications. Three patients (11.1%) were noted to have device‐related thrombus and were treated with an extended course of OAC therapy.
Discussion
In patients with AF, stroke is a devastating complication. Oral anticoagulation with warfarin—and more recently with non–vitamin K antagonist oral anticoagulation—has been the mainstay of treatment for preventing stroke in these patients. While effective for preventing stroke in high‐risk patients, warfarin comes with the risk of drug interactions, the need for frequent monitoring and dose adjustment, and increased potential for bleeding. Non–vitamin K antagonist oral anticoagulants also increase the risk of bleeding, especially in patients who are already at a baseline level of increased risk. Bleeding complications are associated with significant morbidity. Patient concerns about these medications and the lack of reversal agents leads to reduced compliance with prescribed anticoagulation therapy.
LAA closure provides another option for stroke prevention in patients with AF. In contrast to the systemic nature of OAC therapy, LAA closure provides local thrombus control. This relatively new technique has advanced considerably over the past decade. Due to the variable nature of LAA anatomy, imaging guidance is essential for LAA closure procedures.
Currently, TEE and fluoroscopy are most commonly used to guide LAA closure. The use of TEE requires additional sedation, and usually the presence of anesthesia personnel during the procedure if general anesthesia is used. ICE is widely used in many transcatheter interventional procedures.5, 6, 7 ICE offers echo imaging without the need for general anesthesia or additional personnel.
In the present series, we performed LAA closure using the Watchman device with ICE instead of TEE guidance. Berti et al reported a series of ICE‐guided LAA closures using the Amplatzer Cardiac Plug.10 They found a good correlation between ICE‐measured ostium size and landing zone measurements and angiographic measurements.
Both the Amplatzer Cardiac Plug and Watchman are designed for LAA closure. Because of the specific device characteristics and construction, the Watchman device is usually deployed a little deeper in the LAA ostium (“true anatomical ostium”) as compared to the Amplatzer Cardiac Plug device, which is deployed at the level of the “echocardiographic ostium.” Additionally, the delivery sheath is advanced deeper into the LAA during Watchman deployment. For these reasons, accordingly, LAA closure requires additional evaluation of the LAA anatomy, including measurement of LAA depth. From our initial experience, ICE‐guided LAA closure using the Watchman device has a high success rate and good procedural outcome. With improvements in LAA closure techniques and more experience, ICE guidance is a reasonable option for the procedure.
The main advantage of ICE guidance is avoidance of general anesthesia. The use of ICE imaging obviates the need for coordination of multiple personnel (echocardiographer, anesthesiologist, and associated support staff). Aside from cost and risks associated with general anesthesia, patients are able to control their breathing, which provides less anatomical movement during the procedure. Performing the procedure in awake patients may also allow for quicker recognition of procedural complications.
Disadvantages of ICE guidance include its limited echo views, invasiveness, and cost. ICE is fixed to a 45° view, which is the main limitation of this imaging tool. While ICE imaging allows for guidance of transseptal puncture and assessment for pericardial effusion, the view from the right atrium or coronary sinus can sometimes be suboptimal for LAA assessment, especially in the enlarged left atria of AF patients. This imaging limitation can often be overcome by placing the ICE catheter directly in the left atrium or in the left upper pulmonary vein. Direct left atrial imaging requires advancing the ICE catheter through the interatrial septum through the transseptal hole.
LAA closure procedures should be carefully planned in the preimplant phase using TEE or computed tomography scanning to evaluate LAA morphology and exclude intracardiac thrombus. In our experience, the combined use of fluoroscopy and ICE during the implant procedure was adequate to guide device selection, system delivery, positioning, placement, and release. Measurements obtained from ICE and TEE are discordant, as are those obtained from angiography and TEE. These differences, however, were not significant enough to impact device selection in our experience. ICE imaging from the right atrium was often adequate for LAA assessment during the procedure. To evaluate for peridevice leak, ICE evaluation was usually sufficient. We relied on angiography combined with ICE to evaluate this. In cases where the right atrial view was suboptimal, imaging from directly within the left atrium or left upper pulmonary vein provided better assessment. Minor peridevice leaks with smaller than 5‐mm jets often resolved during follow‐up.9
Use of ICE during the procedure requires an additional venous puncture for introduction of the ICE catheter, and ICE imaging does have a learning curve. Venous punctures are well managed in most catheterization labs. Using ICE also avoids the potential risk of esophageal or throat injury associated with TEE.
While the ICE catheter adds additional cost to the procedure, savings from avoidance of general anesthesia and additional personnel may make it more cost effective. Additional savings may be seen with reducing the length of hospital stays and procedure time.
TEE has been the “gold standard” for guiding LAA closure. As ICE advances technically and becomes more commonly used with structural interventions, it may become more widely used to guide LAA closure.
Study Limitations
There were several limitations in our present study. This single‐center study was small and nonrandomized. There was no control group. Patients still underwent TEE imaging prior to the implant procedure. Also, the procedures were performed only by experienced investigators. Further study of this technique is needed.
Conclusions
LAA closure with the Watchman device is feasible and safe under ICE guidance.
Disclosures
Reddy, MD and Neuzil, MD are consultants for Boston Scientific and Biosense Webster Inc. The other authors have no conflicts of interest to declare.
(J Am Heart Assoc. 2016;5:e003695 doi: 10.1161/JAHA.116.003695)
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2. Sherman DG, Goldman L, Whiting RB, Jurgensen K, Kaste M, Easton JD. Thromboembolism in patients with atrial fibrillation. Arch Neurol. 1984;41:708–710. [DOI] [PubMed] [Google Scholar]
- 3. Reddy VY, Holmes DR Jr, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman left appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the continued access registry. Circulation. 2011;123:417–424. [DOI] [PubMed] [Google Scholar]
- 4. Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation for the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Mitchel JF, Gillam LD, Sanzobrino BW, Hirst JA, McKay RG. Intracardiac ultrasound imaging during transseptal catheterization. Chest. 1995;108:104–108. [DOI] [PubMed] [Google Scholar]
- 6. Hijazi Z, Wang Z, Cao Q, Koenig P, Waight D, Lang R. Transcatheter closure of atrial septal defects and patent foramen ovale under intracardiac echocardiographic guidance: feasibility and comparison with transesophageal echocardiography. Catheter Cardiovasc Interv. 2001;52:194–199. [DOI] [PubMed] [Google Scholar]
- 7. Chu E, Kalman JM, Kwasman MA, Jue JC, Fitzgerald PJ, Epstein LM, Schiller NB, Yock PG, Lesh MD. Intracardiac echocardiography during radiofrequency catheter ablation of cardiac arrhythmias in humans. J Am Coll Cardiol. 1994;24:1351–1357. [DOI] [PubMed] [Google Scholar]
- 8. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG) . 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 9. Chue CD, Giovanni JD, Steeds RP. The role of echocardiography in percutaneous left atrial appendage occlusion. Eur J Echocardiogr. 2011;12:i3–i10. [DOI] [PubMed] [Google Scholar]
- 10. Berti S, Paradossi U, Meucci F, Trianni G, Tzikas A, Rezzaghi M, Stolkova M, Palmieri C, Mori F, Santoro G. Periprocedural intracardiac echocardiography for left atrial appendage closure: a dual‐center experience. JACC Cardiovasc Interv. 2014;7:1036–1044. [DOI] [PubMed] [Google Scholar]


