Figure 3.
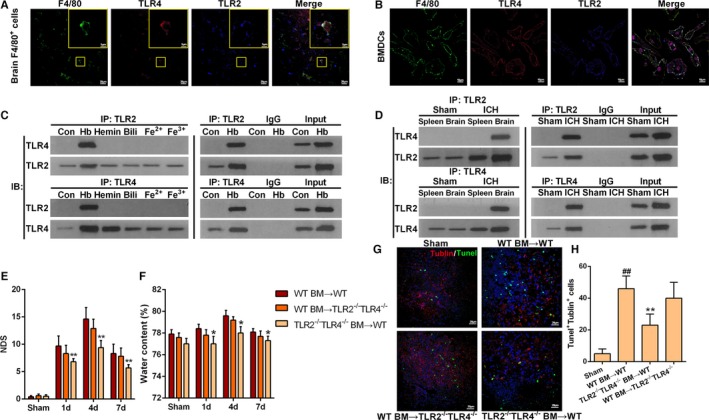
Hemoglobin (Hb)‐induced toll‐like receptor (TLR) 2/4 heterodimer formation on infiltrating macrophages. A, Representative immunofluorescence staining images showing colocalization of TLR2 and TLR4 in F4/80+ cells of perihematomal tissues at 1 day after intracerebral hemorrhage (ICH; scale bars=20 μm). B, Representative immunofluorescence staining images showing colocalization of TLR2 and TLR4 in cultured bone marrow (BM)–derived dendritic cells (BMDCs) after stimulation with Hb (5 μmol/L) for 3 hours (scale bars=10 μm). C, Coprecipitation of TLR2 and TLR4 on cultured BMDCs stimulated with components of red blood cells including Hb, hemin, bilirubin (Bili), Fe2+, and Fe3+, each at a concentration of 5 μmol/L for 3 hours (n=3). D, Coprecipitation of TLR2 and TLR4 on brain infiltrating macrophages or macrophages separated from spleen at 1 day after ICH. Data were obtained for samples pooled from 10 mice, and the experiments were repeated 3 times. E, Neurologic deficit score (NDS) for BM‐chimeric mice analyzed at 1, 4, and 7 days after ICH. TLR2−/−/TLR4−/− BM→WT represents transfer of TLR2/TLR4 double‐knockout BM cells into wild‐type (WT) mice. *P<0.05 vs WT BM→TLR2−/−/TLR4−/− mice, n=4. Two‐way ANOVA reported significant difference in main effects of BM‐chimeric (P<0.05) but not of time points (P>0.05), there was no interaction between BM‐chiemeric and time points (P>0.05). F, Brain water content for BM‐chimeric mice analyzed at 1, 4, and 7 days after ICH. *P<0.05 vs WT BM→TLR2−/−/TLR4−/− mice, n=4. G, Representative immunofluorescence staining images showing βIII tubulin and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) double‐positive cells in the perihematoma area. Brain sections obtained on day 4 after ICH (red=tubulin, green=TUNEL, blue=4′‐6‐diamidino‐2‐phenylindole [DAPI], scale bars=20 μm). H, Quantification of βIII tubulin and TUNEL double‐positive cells at 4 days after ICH. *P<0.05 vs WT, ## P<0.01 vs sham, n=5.
