Abstract
Background:
Malnutrition is common in patients with end-stage renal disease (ESRD) who on peritoneal dialysis (PD) or hemodialysis (HD). This study aimed to compare the frequency distribution of malnutrition in HD and PD patients and its relationship with echocardiographic findings.
Materials and Methods:
This is a case–control study. Using the simple random sampling, 109 patients were selected among HD and PD patients based on the inclusion criteria. HD and PD groups included 55 and 54 patients, respectively. The malnutrition-inflammation score (MIS) index was used to assess malnutrition. Echocardiography was performed by a cardiologist. All the data were analyzed by SPSS version 18.
Results:
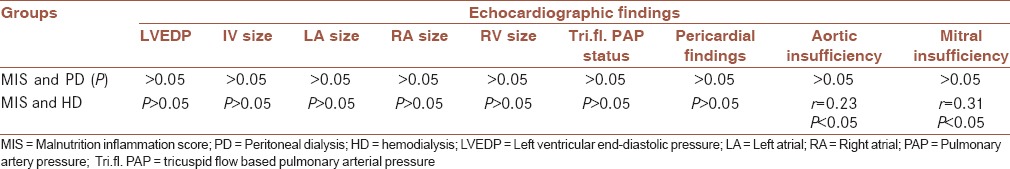
In this study, 79.6% (43 patients) were in the PD group with MIS <9 (no malnutrition to mild malnutrition) and 20.4% (11 patients) with 9 ≤ MIS ≤ 18 suffered from moderate malnutrition. In the HD group, 72.7% (forty patients) had MIS < 9, 25.5% (14) had 9 ≤ MIS ≤ 18, and 1.8% (one patient) with MIS > 18 suffered from severe malnutrition (P = 0.74). There was no significant relationship between MIS and echocardiographic findings in PD patients (P > 0.05). In the HD group, there was no significant relationship between MIS and echocardiographic findings (P > 0.05), except for aortic and mitral valve insufficiencies (P < 0.05).
Conclusion:
The findings of this study show 27.3% of HD patients had moderate to severe malnutrition. There was a statistically significant relationship between MIS index and aortic and mitral valve insufficiencies in HD patients.
Keywords: Continuous ambulatory peritoneal dialysis, end-stage renal disease, hemodialysis, malnutrition-inflammation score
INTRODUCTION
Malnutrition is common in patients with end-stage renal disease (ESRD) who on peritoneal dialysis (PD) or hemodialysis (HD). The causes of malnutrition in HD and PD patients include low energy intake induced by anorexia, loss of nutrients due to dialysis, dietary restriction before dialysis, increased protein catabolism and acidosis.[1,2,3] The need for protein increases in PD patients due to severe peritoneal protein loss which is aggravated due to peritoneal infections.[4,5,6,7,8] In addition to the foregoing, inflammation and inflammatory factors also play an important role in the development of malnutrition in these patients. Some complications of prolonged inflammatory process include protein loss, adipose and muscle atrophy, increased catabolism, oxidative stress, and atherosclerosis.[9,10,11,12,13] Following the deterioration of the nutritional status, ESRD results in inflammatory responses with various mechanisms such as decreased clearance of proinflammatory cytokines, release of oxidative substances such as free oxygen radicals and reduced intake of antioxidants such as Vitamin E.[12,14,15,16,17,18] Recent studies have shown that patients suffering from malnutrition have a higher rate of mortality compared to well-nutritioned patients.[1,2,5,6,7] The major causes of mortality in ESRD patients are cardiovascular complications.[11,19,20] The risk of cardiovascular diseases among HD patients with protein-energy malnutrition (PEM) is higher compared to well-nourished HD patients. Traditional risk factors for cardiovascular diseases such as a high body mass index (BMI) and serum total cholesterol cannot explain the high prevalence of cardiovascular diseases in HD patients with PEM.[17] The most probable mechanism of this complication is myocardial ischemia. Nevertheless, heart failure is an important cause of mortality and morbidity in ESRD patients.[21,22] The prevalence of hypertension, left ventricular hypertrophy, ischemic heart disease, and heart failure is higher in patients with chronic renal failure on dialysis. Their cardiovascular mortality is about 10–30 times more than normal populations.[23,24] In one study, the prevalence of some cardiovascular disease in ESRD patients includes 14% coronary artery disease, 19% angina pectoris, 31% cardiac failure, 7% dysrhythmia, and 8% peripheral vascular disease. On echocardiography 15% had systolic dysfunction, 32% left ventricular dilatation, and 74% left ventricular hypertrophy.[25,26,27,28] According to above studies, cardiovascular complications have been reported in dialysis patients. However, no study has evaluated the relationship of echocardiographic findings and malnutrition in dialysis patients. Therefore, we compared the frequency distribution of malnutrition between hemodialysis and peritoneal dialysis patients and its relationship with echocardiographic findings.
MATERIALS AND METHODS
This case–control study was done in a dialysis center, both HD and continuous ambulatory PD, in 2011–2012 in Isfahan University of Medical Sciences, Iran. HD and PD patients during the study were included in the study. The exclusion criteria included: patients with severe infection during 1 month before our study, change of dialysis method, history of rheumatic heart disease, cardiomyopathy, and primary pulmonary hypertension were not included. A total of 109 participants were selected using the simple random sampling. HD and PD groups included 55 and 54 patients, respectively. Malnutrition-inflammation score (MIS) index was used for the evaluation of malnutrition. MIS consists of ten components, each with four levels of intensity, from 0 (normal) to 3 (severe). The ten components include dry weight change, dietary intake, gastrointestinal symptoms, work capacity, associated diseases, reduced fat stores or loss of subcutaneous fat, reduced muscle mass symptoms, BMI, serum albumin, and total iron-binding capacity levels. The sum of scores ranged from 0 (normal) to 30 (severe malnutrition). According to studies, the grading of severity of malnutrition according to MIS score is as follows:
Lack of malnutrition to mild malnutrition: MIS <9
Moderate malnutrition: MIS = 9–18
Severe malnutrition: MIS >18.
Echocardiography was performed by a cardiologist by Ge vivid 3.
The patient's demographic information including age and sex along with their echocardiographic information such as ejection fraction, regurgitation of aortic, mitral, tricuspid, pulmonary valves, and MIS were recorded. All the data were analyzed by SPSS version 18.0, Inc, Chicago, Ill., USA using descriptive statistics (mean and standard deviation of variables) and Chi-square tests to compare MIS based on the type of dialysis and echocardiographic findings and Mann–Whitney U-test. P < 0.05 was considered statistically significant.
RESULTS
Among the 109 participants, 54 patients were in the PD group. Thirty-nine patients (72.2%) were men and 15 (27.8%) were women with a mean age of 59.77 ± 1.33. In addition, 55 patients were in the HD group. Thirty-five patients (63.60%) were men and 20 (36.4%) were women with the mean age of 54.21 ± 1.30.
Table 1 shows the frequency distribution of MIS in PD and HD groups and compares them.
Table 1.
The frequency distribution of the malnutrition-inflammation score index
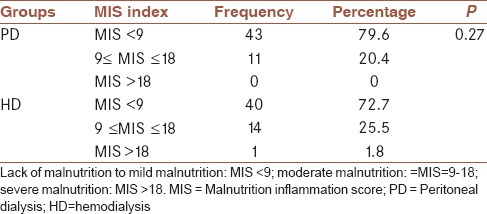
There was no significant difference between the two groups in terms of the frequency distribution of malnutrition indicators (MIS) (P > 0.05).
The relationship between the type of dialysis and echocardiographic findings were analyzed using Chi-squared test.
In the HD group, there was no significant relationship between MIS and echocardiographic findings (P > 0.05), except for aortic and mitral valve insufficiencies (P < 0.05).
Table 2 indicates that there was no significant relationship between MIS and echocardiographic findings in PD patients (P > 0.05).
Table 2.
The relationship between malnutrition-inflammation score and echocardiographic findings in hemodialysis and peritoneal dialysis groups
DISCUSSION
PEM and inflammation are common and usually concurrent in maintenance dialysis patients. Many factors that appear to lead to these two conditions overlap, as do assessment tools and such criteria for detecting them as hypoalbuminemia. Both these conditions are related to poor dialysis outcome. Low appetite and a hypercatabolic state are among common features. PEM in dialysis patients has been suggested to be secondary to inflammation; however, the evidence is not conclusive, and an equicausal status or even opposite causal direction is possible. Hence, malnutrition inflammation score (MIS) is an appropriate term. Possible causes of MIS include comorbid illnesses, oxidative and carbonyl stress, nutrient loss through dialysis, anorexia and low nutrient intake, uremic toxins, decreased clearance of inflammatory cytokines, volume overload, and dialysis-related factors. MIS is believed to be the main cause of erythropoietin hyporesponsiveness, high rate of cardiovascular atherosclerotic disease, decreased the quality of life and increased mortality and hospitalization in dialysis patients. Because MIS leads to a low BMI, hypocholesterolemia, hypocreatininemia, and hypohomocysteinemia, a “reverse epidemiology” of cardiovascular risks can occur in dialysis patients. Therefore, obesity, hypercholesterolemia, and increased blood levels of creatinine and homocysteine appear to be protective and paradoxically associated with a better outcome. There is no consensus about how to determine the degree of severity of MIS or how to manage it. Several diagnostic tools and treatment modalities are discussed. Successful management of MIS may ameliorate the cardiovascular epidemic and poor outcome in dialysis patients.[17,29,30,31] In the comparison between the frequency distribution of MIS in the participants of PD and HD groups, despite the 1.8% participants with severe malnutrition in the HD group, no statistically significant differences was observed. This indicates that MIS was the same in these two groups. Although it was calculated in this study that there is no significant relationship between MIS and echocardiographic findings (ejection fraction, aortic, mitral, pulmonary, and tricuspid valve insufficiencies) in PD participants, there was a significant relationship between MIS and echocardiographic findings and aortic and mitral valve insufficiencies, and other echocardiographic findings had no significant relationship. The study by Liu et al. who evaluated peripheral vascular disease in HD patients, found that the rate of cardiac death, death due to infection, peripheral vascular disease, and cardiovascular hospitalization was higher in them compared to healthy participants.[25] In another study, by Pecoits-Filho et al., it was found that the risk of diastolic heart failure was higher in patients with chronic renal failure compared to the general population.[32]
CONCLUSION
It can be concluded that if severe malnutrition aggravates in HD patients, the chance of a significant relationship between MIS and echocardiographic findings increases.
Finally, this project is recommended to be conducted with a lager sample size to repeat these results so that they are proven with greater certainty.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
AEN contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
AK contributed in the conception of the work, collecting data, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
MA contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work
GA contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
FM contributed in the conception of the work, analyzing data, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
We would like to express thanks for Isfahan Kidney Diseases Research Center.
REFERENCES
- 1.Bossola M, Muscaritoli M, Tazza L, Giungi S, Tortorelli A, Rossi Fanelli F, et al. Malnutrition in hemodialysis patients: What therapy? Am J Kidney Dis. 2005;46:371–86. doi: 10.1053/j.ajkd.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953–60. doi: 10.1093/ndt/15.7.953. [DOI] [PubMed] [Google Scholar]
- 3.Mardani M, Rezapour P, Baba H, Balavar S, Naghdi N. The nutritional status of hemodialysis patients admitted to Khoramabad's Shohadie Ashaier hospital, Korramabad, Iran. J Prev Epidemiol. 2016;1:e09. [Google Scholar]
- 4.Diamond SM, Henrich WL. Nutrition and peritoneal dialysis. In: Mitch WE, Klahr S, editors. Nutrition and the Kidney. Boston: Little, Brown; 1988. pp. 198–223. [Google Scholar]
- 5.Fallahzadeh MH, Fallahzadeh MA. On the occasion of world kidney day 2016; renal disease in children. Acta Persica Pathophysiol. 2016;1:e04. [Google Scholar]
- 6.Marckmann P. Nutritional status of patients on hemodialysis and peritoneal dialysis. Clin Nephrol. 1988;29:75–8. [PubMed] [Google Scholar]
- 7.Amiri M, Hosseini SM. Diabetes mellitus type 1; is it a global challenge? Acta Epidemioendocrinol. 2016;1:e02. [Google Scholar]
- 8.Young GA, Kopple JD, Lindholm B, Vonesh EF, De Vecchi A, Scalamogna A, et al. Nutritional assessment of continuous ambulatory peritoneal dialysis patients: An international study. Am J Kidney Dis. 1991;17:462–71. doi: 10.1016/s0272-6386(12)80642-1. [DOI] [PubMed] [Google Scholar]
- 9.Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17:1085–92. doi: 10.1093/ndt/17.6.1085. [DOI] [PubMed] [Google Scholar]
- 10.Dehghan Shahreza F. Vascular protection by herbal antioxidants; recent views and new concepts. J Prev Epidemiol. 2016;1:e05. [Google Scholar]
- 11.Dehghan Shahreza F. From oxidative stress to endothelial cell dysfunction. J Prev Epidemiol. 2016;1:e04. [Google Scholar]
- 12.Yýlmaz MY. The causes of the inflammation and possible therapeutic options in dialysis patients. Gulhane Med J. 2007;49:271–6. [Google Scholar]
- 13.Afshar R, Sanavi S, Izadi-Khah A. Assessment of nutritional status in patients undergoing maintenance hemodialysis: A single-center study from Iran. Saudi J Kidney Dis Transpl. 2007;18:397–404. [PubMed] [Google Scholar]
- 14.Khazaei M. Adipokines and their role in chronic kidney disease. J Nephropharmacol. 2016;5:69–70. [PMC free article] [PubMed] [Google Scholar]
- 15.Enia G, Sicuso C, Alati G, Zoccali C. Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant. 1993;8:1094–8. [PubMed] [Google Scholar]
- 16.Duerksen DR, Yeo TA, Siemens JL, O’Connor MP. The validity and reproducibility of clinical assessment of nutritional status in the elderly. Nutrition. 2000;16:740–4. doi: 10.1016/s0899-9007(00)00398-1. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am J Kidney Dis. 2003;42:864–81. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Lala MA, Nazar CM, Lala HA, Singh JK. Interrelation between blood pressure and diabetes. J Ren Endocrinol. 2015;1:e05. [Google Scholar]
- 19.Lal AK, de Biasi AR, Alexander S, Rosenthal DN, Sutherland SM. End-stage renal disease and cardiomyopathy in children: Cardiac effects of renal transplantation. Transplantation. 2012;93:182–7. doi: 10.1097/TP.0b013e31823be7f8. [DOI] [PubMed] [Google Scholar]
- 20.Dehghan Shahreza F. Mechanistic impact of renal tubular cell protection by antioxidants. Ann Res Antioxid. 2016;1:e06. [Google Scholar]
- 21.Galli EG, Taietti C. Home peritoneal ultrafiltration in the treatment of chronic heart failure. G Ital Nefrol. 2011;28:506–13. [PubMed] [Google Scholar]
- 22.Nazar CM, Bashir F, Izhar S, Ahmed SA. Does frequent hemodialysis regimen result in regression of left ventricular mass compared to conventional hemodialysis? J Nephropharmacol. 2015;4:37–41. [PMC free article] [PubMed] [Google Scholar]
- 23.Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, et al. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12:1508–15. doi: 10.1681/ASN.V1271508. [DOI] [PubMed] [Google Scholar]
- 24.Momeni A. Cardiovascular complications of renal failure in hemodialysis patients. Ann Res Dial. 2016;1:e05. [Google Scholar]
- 25.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–92. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 26.Nasri H, Ardalan MR, Rafieian-Kopaei M. On the occasion of world hypertension day 2014. J Parathyr Dis. 2014;2:5–6. [Google Scholar]
- 27.Rastegari F. The healthy diet for cardiovascular disease. Acta Persica Pathophysiol. 2016;1:e02. [Google Scholar]
- 28.Hajian S. Positive effect of antioxidants on immune system. Immunopathol Persa. 2015;1:e02. [Google Scholar]
- 29.Roozbeh J, Sagheb MM, Vafaie E. The association between blood pressure level and serum uric acid concentration in hemodialysis patients. J Nephropathol. 2015;4:85–90. doi: 10.12860/jnp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pecoits-Filho R, Bucharles S, Barberato SH. Diastolic heart failure in dialysis patients: Mechanisms, diagnostic approach, and treatment. Semin Dial. 2012;25:35–41. doi: 10.1111/j.1525-139X.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 31.Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev. 2015;4:28–33. doi: 10.12861/jrip.2015.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Liang KV, Rosenbaum A, Stephenson R, Pike F, Weissfeld L. Peripheral vascular disease severity impacts health outcomes and health-related quality of life in maintenance hemodialysis patients in the HEMO Study. Nephrol Dial Transplant. 2012;27(7):2929–36. doi: 10.1093/ndt/gfr760. [DOI] [PubMed] [Google Scholar]


