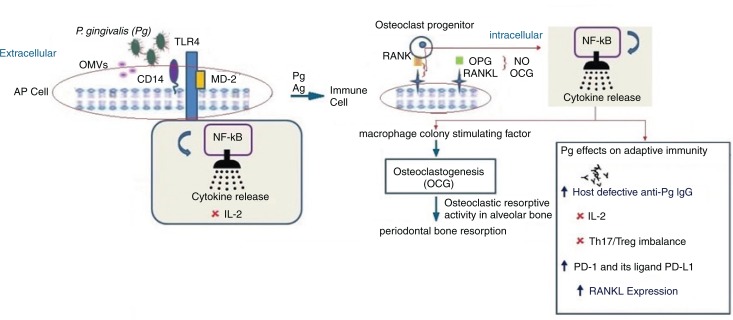
Fig. 1.
P. gingivalis and its presumed contribution to periodontitis via adaptive immune suppression. Following P. gingivalis infection, the outer membrane vesicles (OMVs) from P. gingivalis transfer LPS and gingipains to a lipid-binding site on an antigen presenting cell (AP cell). Gingipains erode cells cleaving CD14 and the immune cell receptor (RANKL [receptor activator of NF-κB ligand]) a TNF-related cytokine which binds to RANK, a protein expressed on the osteoclast progenitor cell activating an intracellular signaling cascade via NF-κB resulting in suppression of IL-2 secretion. Intact CD14 on membrane and soluble TLR4-MD2 (myeloid MD2= differentiation protein 2) can still function to promote the binding of LPS to the TLR4-MD2 complex and enlist intracellular cell signaling via NF-κB. The appropriate cytokine release or its suppression has implications on cellular/adaptive immune responses which involve host defective IgG. Adaptive immune responses through activated CD14 T cells and secretion of immunoglobulins (Ig) by B cells constrain the subgingival biofilm or may give rise to disease progression. T cells can have both protective and destructive roles. Inhibition of IL-2: P. gingivalis can modify adaptive immune response through interaction with dendritic cells inducing a cytokine pattern favoring T-helper 17 (Th17) lineage and inhibiting the expression and accumulation of IL-2 which attenuates T cell proliferation and communication. Arg-gingipain (Agp) protease is involved in suppression of IL-2 which contributes to self-propagation of P. gingivalis in vivo. Th17/Treg imbalance: P. gingivalis can modify adaptive immune response by interaction with dendritic or antigen presenting cells (APC cells) which induce a cytokine pattern favoring the Th17 cell population. The imbalance is also promoted by P. gingivalis proteases because IL-1β is the most Th17 supporting cytokine and is the cytokine most resistant to P. gingivalis protease cleavage modification of Th17/Treg balance which occurs by Th17 cell response inhibition and increasing Treg-cell activation. IFN-γ suppresses upregulation of programmed cell death: Secretion of IFN-γ upregulation of programmed cell death – 1 (PD-1) – receptor on CD+ cells and its ligand PD-L1 on CD11b+-T cells. Since the PD-L1/PD-1 signaling pathway inhibits the T-cell response, the changes induced by P. gingivalis on the expression of these molecules could be a mechanism by which P. gingivalis suppresses T-cell immunity. RANK-RANKL, OPG: Activated lymphocytes expressing surface receptor activator of NF-κB ligand (RANKL) can bind to surface RANK expressed on hematopoietic progenitors of osteoclasts (osteoclast progenitors) activating a signal transduction cascade leading to osteoclastogenesis (in the presence of macrophage colony–stimulating factor, MCSF). This gives rise to activation and differentiation of functional osteoclasts and periodontal bone resorption. Osteoprotegerin (OPG), an inhibitor of RANKL–RANK interaction, produced by gingival fibroblasts, osteoblasts, and periodontal ligament fibroblasts, abrogates immune cell RANKL-dependent and destructive osteoclastic periodontal bone resorption. OPG can enhance bone formation.
Symbols:  =suppression,
=suppression,  =upregulated,
=upregulated,  =leads to,
=leads to,  =contribution from, toll-like receptor 4 (TLR 4) and
=contribution from, toll-like receptor 4 (TLR 4) and  =from the osteoclast cell-surface receptor (RANK) and its membrane-bound ligand,
=from the osteoclast cell-surface receptor (RANK) and its membrane-bound ligand,  =mRANKL or sRANKL (Receptor activator of nuclear factor-κB ligand),
=mRANKL or sRANKL (Receptor activator of nuclear factor-κB ligand),  =P. gingivalis,
=P. gingivalis,  =antibodies to P. gingivalis,
=antibodies to P. gingivalis,  =outer membrane vesicles (OMVs),
=outer membrane vesicles (OMVs),  =release of cytokines,
=release of cytokines,  =osteoprotegerin (OPG),
=osteoprotegerin (OPG),  =cell-surface receptor CD14,
=cell-surface receptor CD14,  =RANK, a receptor expressed on the cell surface of osteoclast progenitor cells.
=RANK, a receptor expressed on the cell surface of osteoclast progenitor cells.