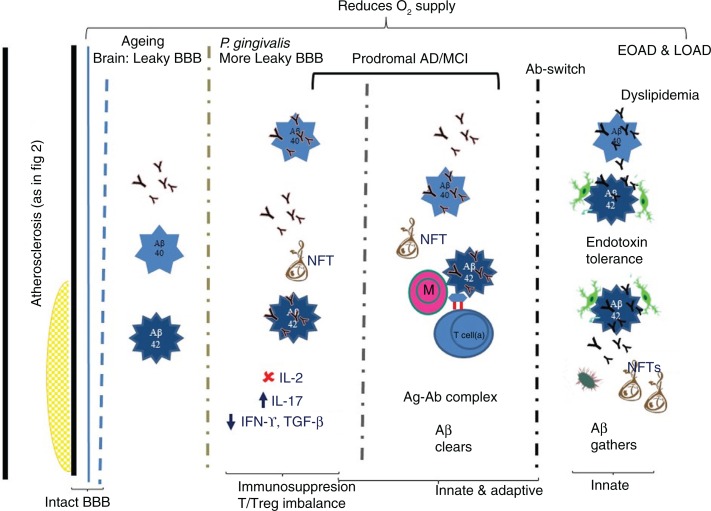
Fig. 3.
P. gingivalis and its presumed contribution to AD via adaptive immune suppression. Vascular integrity with atherosclerotic plaque formation compromises blood flow and the available oxygen. Th17/Treg imbalance leads to immunosuppression. Insufficient cytokine levels (TGF-β and IFN-γ) mean that neurons in the hippocampal dentate gyrus cannot regenerate. During advancing age the blood-brain barrier (BBB) becomes leaky and if P. gingivalis infection persists, BBB becomes even more permeable. The greater opening of the BBB allows entry of larger plasma proteins into the brain. During aging, the brain shows largely Aβ1-40 deposition and few neurofibrillary tangles (NFTs). In early (prodromal/EOAD) and late-onset (LOAD) AD, copious amounts of Aβ1-40/1-42 deposits and NFTs occur. It is hypothesized that in prodromal/EOAD, Aβ deposits associate with few migrated monocytes and Tregs and functional antibodies. These are able to form antigen-antibody complexes (Ag-Ab complex) to clear Aβ deposits. In LOAD, an antibody switch takes place (IFN-γ imbalance), during which non-functional antibody is secreted, which binds Aβ, but does not promote its clearance by activated microglia from the brain. More NFTs also accumulate.