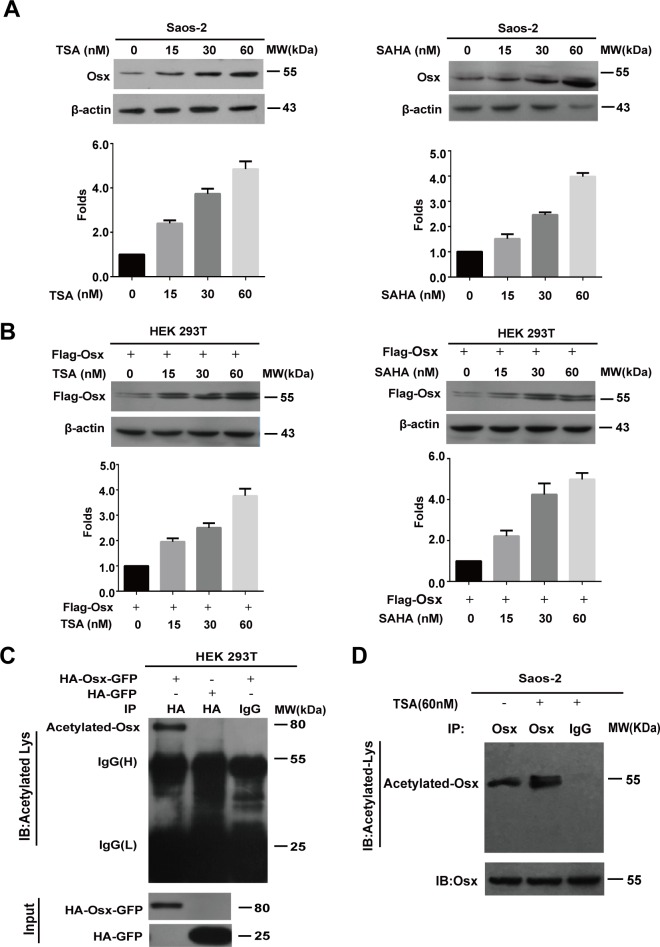
Figure 1. The Osx protein is acetylated.
A. Saos-2 cells were treated with TSA (0, 15, 30, 60 nM) or SAHA (0, 15, 30, 60 nM) for 24 h. Endogenous Osx protein was detected by western blotting with an anti-Osx antibody. β-Actin served as a loading control. The protein levels of Osx in the upper panel were determined by densitometry (lower). B. HEK 293T cells were transfected with Flag-Osx plasmids and then treated with TSA (0, 15, 30, 60 nM) or SAHA (0, 15, 30, 60 nM) for 24 h. Exogenous Osx protein was detected by western blotting with an anti-Flag antibody. β-Actin served as a loading control. The protein levels of Osx in upper panel were determined by densitometry (lower panel). C. HEK 293T cells were transiently transfected with HA-Osx-GFP or HA-GFP expression plasmids. The cell lysates were immunoprecipitated with an anti-HA antibody and then blotted with an anti-Acetylated-Lys antibody. HA-Osx-GFP and HA-GFP protein were detected by western blotting analysis with an anti-HA antibody. D. Saos-2 cells were treated with or without TSA (60 nM) for 24 h. The cell lysates were immunoprecipitated with an anti-Osx antibody (rabbit polyclonal antibody) and then blotted with an anti-Acetylated-Lysine antibody (mouse monoclonal antibody). Osx protein was detected by western blotting analysis with an anti-Osx antibody. Results are shown for one of three independent experiments.