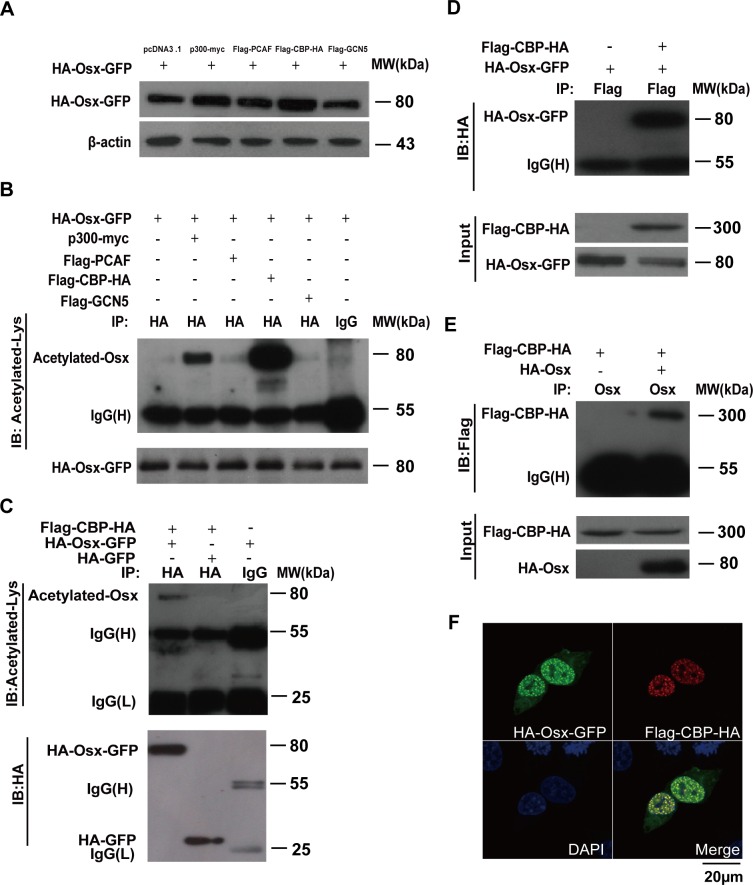
Figure 2. Osx acetylation is mediated by CBP/p300.
A. HEK 293T cells were transiently co-transfected with HA-Osx-GFP and p300-myc, Flag-PCAF, Flag-CBP-HA, or Flag-GCN5 expression vectors. pcDNA3.1 empty vector co-transfection was used as a control. The Osx protein was detected by western blotting with an anti-HA antibody. β-Actin served as a loading control. B. The cell lysates of the above-transfected cells were immunoprecipitated with an anti-HA antibody and then blotted with an anti-Acetylated-Lys antibody. HA-Osx-GFP protein was detected by western blotting with an anti-HA antibody. C. HEK 293T cells were transiently co-transfected with Flag-CBP-HA and HA-Osx-GFP or HA-GFP expression plasmids, the cell lysates were immunoprecipitated with an anti-HA antibody and then blotted with an anti-Acetylated-Lys antibody. HA-Osx-GFP and HA-GFP protein were detected by western blotting with an anti-HA antibody. D. HEK 293T cells were transiently transfected with HA-Osx-GFP alone or together with the Flag-CBP-HA expression plasmid, the cell lysates were immunoprecipitated with an anti-Flag antibody and then blotted with an anti-HA antibody. Flag-CBP-HA and HA-Osx-GFP protein were detected by western blotting with an anti-HA antibody. E. HEK 293T cells were transiently transfected with Flag-CBP-HA alone or together with HA-Osx expression plasmid, the cell lysates were immunoprecipitated with an anti-Osx antibody and then blotted with an anti-Flag antibody. Flag-CBP-HA and HA-Osx protein were detected by western blotting with an anti-HA antibody. F. HEK 293T cells were transiently co-transfected with HA-Osx-GFP and Flag-CBP-HA expression plasmids. The localization of Osx was visualized as green fluorescence, and CBP was visualized by immunostaining with an anti-Flag antibody (red). Nuclei were visualized using 4, 6-diamidino-2-phenylindole (DAPI) staining (blue). Experiments were repeated at least three times.