Figure 2. Sparse deletion of Neuroligins from mature hippocampal CA1 pyramidal neurons in juvenile mice causes similar but less severe phenotypes as deletions in developing neurons.
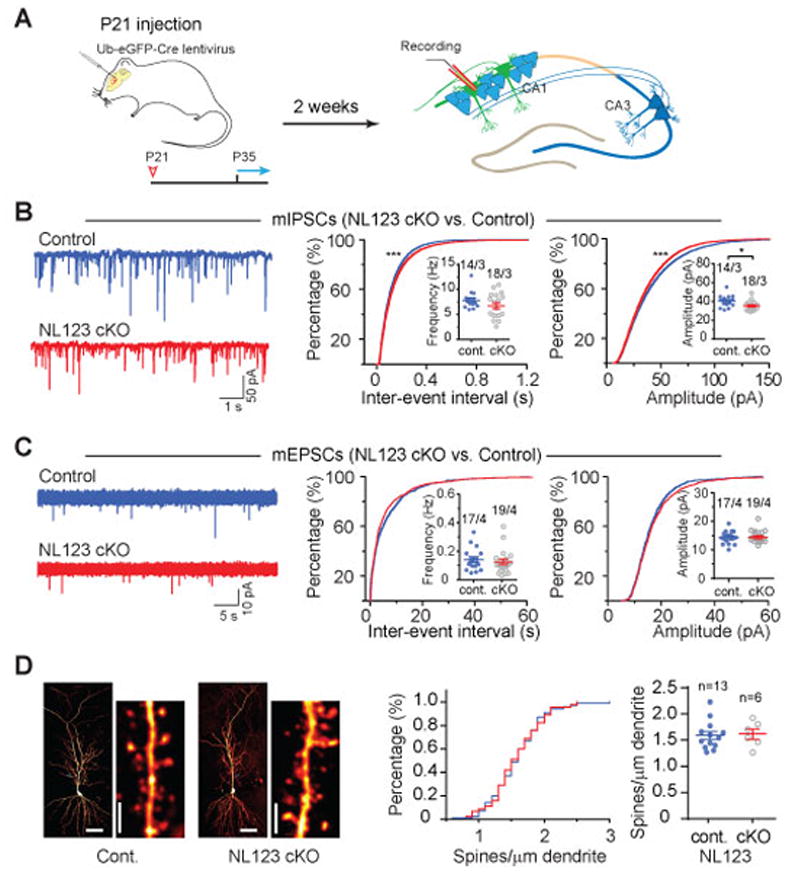
(A) Experimental design for injections at P21, followed by experimental analyses from P35 onwards.
(B) Analysis of mIPSCs in triple NL123 cKO neurons. Left, representative traces; middle, cumulative distribution of the mIPSC inter-event interval (inset: data points from individual cells and means of mIPSC frequency); right, cumulative distribution of mIPSC amplitudes (inset: data points from individual cells and mean mIPSC amplitudes).
(C) Same as B, but for mEPSCs.
(D) NL123 deletion in juvenile mice also does not change the spine density of CA1 pyramidal cells. Left, representative images of biocytin-stained CA1 pyramidal neurons after patch-clamp recording, with a lower and higher magnification images shown side by side (calibration bars: 50 μm and 2 μm, respectively); middle, cumulative distribution of spine density (control: 100 dendrites/13 neurons; cKO: 45 dendrites/6 NL123 cKO pyramidal neurons); right, summary graph of mean spine densities with data points from individual neurons.
Data in summary graphs are means ± SEM; statistical comparisons were performed with the Kolmogorov-Smirnov test (cumulative distributions) or student’s t-test (*, p<0.05; **, p<0.01; ***, p<0.001; non-significant comparisons are not labeled). Numbers indicate number of cells/mice examined.
