Abstract
Rationale: Primary ciliary dyskinesia (PCD) is a rare disease. There are no available data on disease-specific pediatric patient–reported outcomes.
Objectives: Our objective was to create developmentally appropriate, health-related quality-of-life questionnaires (QOL-PCD) for children (6–12 yr) and adolescents (13–17 yr) with PCD and a parent proxy measure.
Methods: The QOL-PCD was developed using a cross-cultural protocol-driven approach satisfying both North American and European drug regulatory agency guidelines. A conceptual framework was generated by literature review, focus groups (expert clinicians and patients/parents), and open-ended interviews with children, adolescents, and parents of patients with PCD. We recruited participants from international research consortiums, PCD clinics, and patient advocacy groups, aiming for representation of a wide spectrum of disease severity, sociodemographic status, and ethnicity. Qualitative interviews were conducted by trained and experienced research assistants and psychologists. Transcripts were content-analyzed with Atlas.ti/NVivo to assess saturation of content. A self-completed item relevance survey was administered to E.U. participants. Qualitative and quantitative data were used to construct draft instruments. Questionnaires were further refined after cognitive interviews.
Measurements and Main Results: Focus groups (n = 62 experts; n = 20 patients/parents) and open-ended interviews with patients/parents (n = 69; 34 males; age at diagnosis, 0–15 yr; FEV1, 58–118% predicted) revealed a wide spectrum of issues unique to this population. Content analysis of transcripts identified the following domains, depending on age: Respiratory Symptoms, Physical Functioning, Emotional Functioning, Treatment Burden, Ears and Hearing, Sinus Symptoms, Social Functioning, Role Functioning, Vitality, Health Perceptions, School Functioning, and Eating and Weight. Various items were retained in questionnaires, based on age and role of respondent: 37, 43, and 41 items for children, adolescents, and parent proxy, respectively. The item relevance survey (n = 57) yielded results similar to those of open-ended interviews. Cognitive testing (n = 47; 20 males; age at diagnosis, 0–11 yr; FEV1, 49–124% predicted) confirmed that items and response choices were clear and understood by respondents, and that all relevant items were included.
Conclusions: The QOL-PCD measures, developed using rigorous, protocol-driven methods and international collaborations, have demonstrated content validity and cross-cultural equivalence for implementation in English-speaking populations. Psychometric testing is underway to determine their measurement properties for evaluating clinical interventions and informing quality of care.
Keywords: primary ciliary dyskinesia, health-related quality of life, patient-reported outcomes, pediatric, parent proxy
Primary ciliary dyskinesia (PCD) is a rare inherited lung disease affecting motile cilia, such that mucociliary clearance is impaired. Individuals with PCD often present with unexplained respiratory symptoms in the first few days of life, develop persistent sinopulmonary symptoms in infancy, bronchiectasis during childhood, and a progressive decline in lung function over time, which can lead to end-stage lung disease by early to mid-adulthood (1). To date, no medications to treat PCD have been approved by regulatory bodies (2), and a major obstacle to monitoring disease progression and evaluating new treatments is the lack of disease-specific outcome measures (3). Current outcome measures, such as spirometry, chest computed tomography, and lung clearance index all have limitations in terms of their sensitivity and feasibility for evaluating new therapies or disease progression (4–7). Importantly, these physiological measures do not reflect the impact of the disease on patients’ daily symptoms or functioning (e.g., physical, respiratory, social) as required by the Food and Drug Administration (FDA) (8) and the European Medicines Agency (EMA) (9, 10).
Thus, measures are needed to assess the impact of PCD, from the patient perspective, on all domains of daily functioning. Health-related quality-of-life (HRQoL) measures are valid, reliable, and informative indices of symptoms and functioning and sensitive to patient concerns (11–14). Both the FDA and EMA support the development and use of disease-specific patient-reported outcome measures for evaluation of new medications and treatments (12). At present there are no validated HRQoL measures available for pediatric patients with PCD.
Developing a new patient-reported outcomes measure requires concept elicitation from patients, using rigorous qualitative methods, to achieve content validity, which is a challenge when patients are geographically disparate with a rare disease. Once a measure with content validity is developed, it can be subjected to psychometric testing to determine its measurement properties (reliability, construct validity, and responsiveness), with item reduction as needed.
Our goal was to develop harmonized (North America and Europe) pediatric PCD-specific HRQoL instruments informed by guidance from the FDA and EMA (8–10), to be used as primary or secondary outcomes in clinical trials. In addition, because patient-reported outcome measures demonstrate optimal reliability and validity when they are specific to the respondent’s developmental stage (15), we developed three separate age-appropriate versions of this instrument: children (aged 6–12 yr), adolescents (aged 13–17 yr), and parent proxy (for children aged 6–12 yr). This article reports on the development process and qualitative research results for these three instruments. Some of the results of this research have been previously reported in the form of an abstract (16).
Methods
Conceptual Framework and Study Design
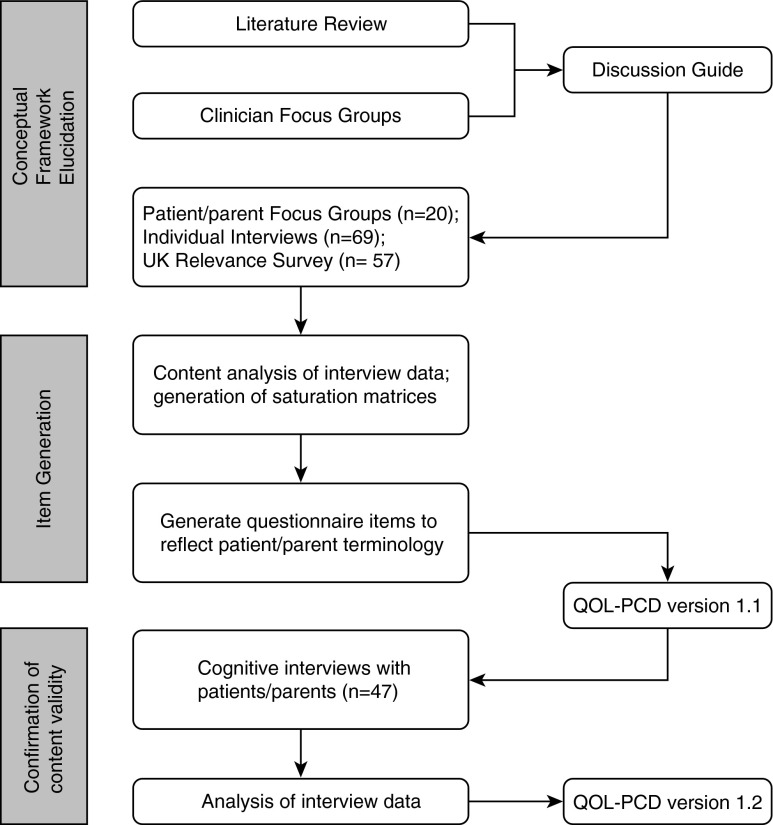
We followed the patient-reported outcomes measure development process outlined by the FDA (8) and EMA (10) to design the study, procedures, and analytic plan (Figure 1). The study was approved by the Research Ethics Board at the Hospital for Sick Children (Toronto, ON, Canada); the Institutional Review Boards at the University of North Carolina (Chapel Hill, NC), Washington University (St. Louis, MO), and the University of Miami (Miami, FL); and the National Research Ethics Service (London, UK) (UK 07/Q1702/109). Informed consent and assent, as appropriate, were obtained before interviews.
Figure 1.
Development process for pediatric health-related quality-of-life measures for primary ciliary dyskinesia (QOL-PCD).
Literature Review and Clinician Focus Groups
First, a systematic literature review was conducted to identify key symptoms and effects of PCD on patient functioning. MEDLINE and EMBASE were searched and additional references were sought through citations in reviewed studies. Next, expert physicians (e.g., pulmonologists, otolaryngologists), allied health professionals, and researchers met to discuss their own perceptions of the impact of PCD on pediatric patients at the American Thoracic Society Conference and European Respiratory Society Congress. These literature reviews and discussions led to the development of the conceptual framework, which guided the open-ended interviews subsequently conducted with patients with PCD and parent caregivers.
Participants with PCD and Setting
English-speaking children with PCD, ages 6–17 years, and parent caregivers, were recruited from the North American (United States and Canada) GDMCC (Genetic Diseases of Mucociliary Clearance Consortium) sites and European (United Kingdom and Ireland) BESTCILIA (Better Experimental Screening and Treatment for Primary Ciliary Dyskinesia) sites during periods of clinical stability. Participants in North America were recruited from a patient conference sponsored by the North American PCD Foundation, and patients with PCD were evaluated at the University of North Carolina, Washington University, and the Hospital for Sick Children.
In Europe, participants were recruited from PCD clinics in the United Kingdom and Ireland and via an announcement circulated through the PCD Family Support Group UK. We used convenience sampling, but aimed to recruit patients of various ethnic backgrounds, socioeconomic status, and disease severity to increase the representativeness of the sample.
To avoid bias associated with diagnostic misclassification, criteria for participation in the open-ended and cognitive interviews included a confirmed diagnosis of PCD through the GDMCC or BESTCILIA specialized PCD research centers. North American participants were diagnosed on the basis of a compatible clinical phenotype plus defect in ciliary ultrastructure and/or identification of biallellic disease-causing mutations in one of the PCD genes (17). UK and Irish participants had a multidisciplinary diagnostic decision based on clinical phenotype, nasal nitric oxide, and reproducible “hallmark” abnormalities of ciliary function assessed by high-speed video analysis and/or assessment of ciliary ultrastructure by electron microscopy (18).
Item Generation: PCD Patient/Parent Focus Groups and Individual Interviews; UK Relevance Survey
We conducted focus groups with children, adolescents, and parents at a PCD Family Day sponsored by the North American PCD Foundation in Buffalo, New York. Individual, semistructured, open-ended interviews were then conducted in person with children and adolescents with PCD and parent caregivers at multiple sites in North America and Europe to elicit the effects of PCD from the patient and parent perspectives. All interviews were conducted in North America by A.L.Q., A.A., and other research assistants, and in the United Kingdom by L.B. All interviewers were psychologists, had extensive training and experience in conducting qualitative interviews, and had no preexisting relationships with the study participants. The framework (interview guide) underpinning the interviews was prospectively and jointly (between North American and European sites) developed after the expert focus groups and literature review.
In addition, a postal survey with a list of potential items generated from the clinician and patient/parent focus groups was circulated by the Family Support Group in the United Kingdom, to rate item relevance and importance by patients. Participants were asked to rate each item on a five-point Likert scale (1, not relevant; 5, highly relevant). Responses were analyzed according to age group, examining means and response distributions for each item for the child, adolescent, and parent samples.
Content Analysis of Patient/Parent Interview Data
All interviews were audiotaped and transcribed for content analysis, using either Atlas.ti in North America (version 7.0; Scientific Software Development, Berlin, Germany) or NVivo in the United Kingdom (version 8.0; QSR International Pty Ltd, Doncaster, Victoria, Australia). Thematic coding was used to identify key symptoms and psychosocial impacts. These data were then analyzed for their frequency of endorsement and level of impact. Saturation matrices were derived to inform item generation and to ensure that data saturation was achieved (i.e., no new themes arose with new interviewees) (19). Frequently endorsed items were written using patient and parent language from the original transcripts. This ensured that the items captured the meaning, language level, and context of their experiences.
Construction of Prototype Questionnaires
Agreement on item selection and wording was achieved during multidisciplinary, multinational conference calls using a modified Delphi approach (20). We discussed the specific quotes and saturation grids from the interviews, and results from the item relevance survey. Selected items were written using patient language and phrases obtained in the qualitative interviews and were then combined into scales based on our conceptual framework (e.g., frequency and severity of respiratory symptoms, perceptions of treatment burden). Items were written to ensure conceptual, cultural, and linguistic equivalence for North America, Ireland, and the United Kingdom by researchers from both regions. We also adhered to both the FDA and EMA guidances (8–10), and used a short recall period (i.e., 1 wk).
Cognitive Testing of Prototype Questionnaires
Cognitive interviews were conducted using a “think aloud” procedure (21) to evaluate the clarity, interpretation, relevance, and comprehensiveness of the draft instruments. In particular, we asked about the instructions, interpretation of items, and use of the rating scales. We also asked whether any relevant items were missing. First, participants completed the prototype questionnaire independently. Next, they were interviewed either in-person or by phone (older adolescents, parents), using specific cognitive probes. All interviews were audio-recorded and transcribed. The results were discussed during a series of teleconferences to determine whether revisions were required in the formatting, instructions, items, or rating scale options. The measures were refined on the basis of these cognitive interviews and finalized to form the draft instruments for psychometric validation.
Results
Conceptual Framework Elucidation
Items were initially generated from the literature review and focus groups with expert clinicians treating PCD in North American (n = 12) and Europe (n = 40). Clinicians outlined a number of symptoms related to respiratory and upper airway pathophysiology, and problems with chronic otitis media and its sequelae (e.g., difficulty hearing, speech delays). A conceptual framework was then developed representing key quality-of-life domains: physical functioning, emotional functioning, treatment burden, symptoms (respiratory, sinonasal, ear and hearing), social functioning, role functioning, eating and weight, and body image (see Appendix E1 in the online supplement).
Three separate focus groups at a North American PCD family day provided information on the effects of PCD from the patient and parent perspectives (n = 9, parents of children under age 12; n = 4 adolescent ages 12 and older; n = 7 parents of adolescents), which facilitated our refinement of the conceptual framework. In addition to symptoms, questions were asked about how PCD impacted physical functioning, energy level, and social and emotional functioning.
Although not as well established as the cystic fibrosis (CF) treatment regimen, a number of treatments are currently prescribed by pulmonologists treating patients with PCD. Therefore, we asked several questions about treatment burden. Social functioning was also affected by embarrassment about coughing, sputum production, and ear drainage. All of these concepts were included in the preliminary conceptual framework underlying the patient/parent open-ended interview guide.
Patient/Parent Individual Interviews and UK Relevance Survey
The North American focus groups were composed of pediatric patients with PCD (n = 9), parents of children under age 12 years (n = 7), and parents of adolescents, ages 12 years and older (n = 4). North American and European Union participants who completed the open-ended interviews included children (n = 20), adolescents (n = 20), and parents (n = 29), representing a wide range of disease severity (FEV1, 58–118% predicted) and ethnic groups (Table 1). Similar numbers of boys and girls participated, but the majority of parent respondents were mothers. As expected, nearly all informants described a chronic cough and sinonasal symptoms. Selected patient quotes from the open-ended interview phase in North America and the European Union are presented in Table 2.
Table 1.
Characteristics of patients with primary ciliary dyskinesia participating in open-ended interviews and cognitive testing
| Open-Ended Interviews |
Cognitive Interviews |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Child |
Parent Proxy |
Adolescent |
Child |
Parent Proxy |
Adolescent |
|||||||
| UK/I (n = 4) | North America (n = 16) | UK/I (n = 6) (9 children) | North America (n = 23) (20 children) | UK/I (n = 4) | North America (n = 16) | UK/I (n = 10) | North America (n = 4) | UK/I (n = 10) | North America (n = 7) (6 children) | UK/I (n = 8) | North America (n = 8) | |
| Participant sex | ||||||||||||
| Male | 3 | 5 | 4 | 10 | 1 | 11 | 3 | 3 | 3 | 2 | 5 | 4 |
| Female | 1 | 11 | 5 | 10 | 3 | 5 | 7 | 1 | 7 | 4 | 3 | 4 |
| Participant age | ||||||||||||
| 6–12 yr | 4 | 15 | 9 | 15 | 0 | 1 | 10 | 4 | 10 | 5 | 0 | 0 |
| 13–17 yr | 0 | 1 | 0 | 5 | 4 | 15 | 0 | 0 | 0 | 1 | 8 | 8 |
| Ethnicity | ||||||||||||
| White | 4 | 14 | 8 | 16 | 4 | 14 | 8 | 3 | 8 | 4 | 8 | 5 |
| Black | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hispanic | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Asian | 0 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 2 | 2 | 0 | 2 |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Missing | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age at diagnosis | ||||||||||||
| Years (SD) | 3 (1) | 3 (2) | 4 (3) | 4 (3) | 4 (2) | 8 (4) | 3 (3) | 6 (5) | 3 (3) | 4 (4) | 5 (3) | 6 (4) |
| Range | 1–4 | 0–7 | 0–8 | 0–10 | 3–7 | 2–15 | 0–7 | 0–11 | 0–8 | 0–11 | 0–10 | 0–11 |
| Time since diagnosis | ||||||||||||
| Years (SD) | 8 (3) | 6 (3) | 4 (2) | 7 (3) | 10 (4) | 7 (4) | 6 (4) | 4 (3) | 8 (3) | 4 (2) | 9 (3) | 9 (4) |
| Range | 4–9 | 3–12 | 1–6 | 4–12 | 5–14 | 1–15 | 2–10 | 0–8 | 3–11 | 2–7 | 5–12 | 2–14 |
| FEV1% predicted | ||||||||||||
| Mean (SD) | 80 (21) | 85 (17) | 87 (9) | 90 (18) | 90 (16) | 96 (17) | 76 (16) | 91 (20) | 86 (16) | 88 (18) | 88 (13) | 91 (17) |
| Range | 59–103 | 58–109 | 75–99 | 58–109 | 68–105 | 73–118 | 49–96 | 69–116 | 67–111 | 69–116 | 73–110 | 69–124 |
| Missing | 0 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| disease characteristics | ||||||||||||
| Chronic cough | 4 | 16 | 8 | 19 | 4 | 16 | 8 | 4 | 10 | 6 | 8 | 8 |
| Chronic nasal congestion | 4 | 16 | 7 | 20 | 4 | 16 | 8 | 4 | 10 | 6 | 7 | 8 |
| Bronchiectasis | 1 | 14 | 2 | 20 | 1 | 12 | 1 | 4 | 2 | 6 | 4 | 8 |
| Situs inversus totalis | 0 | 7 | 5 | 10 | 2 | 5 | 4 | 1 | 2 | 1 | 2 | 3 |
| Situs ambiguous | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac disease | 0 | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
Definition of abbreviation: UK/I = UK and Ireland.
Table 2.
Sample quotations from primary ciliary dyskinesia patient/parent open-ended interviews
| Topic | Quote |
||
|---|---|---|---|
| Child | Adolescent | Parent Proxy | |
| Physical functioning | “…stops me from running as fast as other people and taking part in sports, and then not having all the senses that I need to know what’s around me.” —Male, UK | “…my immune system…is already not very strong and…I can’t get as much oxygen in as the other kids. Meaning I can’t keep up with them in sports or anything.” —Male, North America | “…if he’s playing in school and …he needs to run around, then he gets more tired than other kids and they’re still running around and he’s stopping.” —Parent, UK |
| Emotional functioning | “It…just wastes all of my energy, it makes me feel like I don’t want to wake up in the mornings.” —Male, UK | “I was sick on and off…it’s just frustration. Because there’s no cure.” —Male, North America | “Sometimes, when he sees his friends running around and he can’t tag them, then he feels like ‘why do I have PCD?’” —Parent, UK |
| Treatment burden | “…you’re having a hard day and it takes too long.” —Male, UK | “it’s kind of annoying really, becau se I have to do it for ten minutes…it takes time out of my life that I want to go off and do something else instead.” —Male, UK | “…having two with the same condition and trying to [get] both of their physios in on a day-to-day basis, making sure that they both take their medication and wear their hearing aids… is quite a challenge.” —Parent, UK |
| “…sometimes I don't want to do my best. I just want to play.” —Male, North America | “…he’s fed up with it sometimes.” —Parent, UK | ||
| Ear and hearing symptoms | “…sometimes I have to ask…people the same questions over and over again before I…start hearing them.” —Male, UK | “…normally when people have ear infections…they have temperatures and they hurt. Well, I've gotten so used to it that I don't even know I have an ear infection.” —Male, North America | “if she has a speech problem or…coughing constantly…when they’re in school, it might become embarrassing.” —Parent, UK |
| “…it’s hard to hear when there’s background noise.” —Female, North America | |||
| Respiratory symptoms | “…I’m coughing every minute... sometimes half a minute, cough, cough, cough, cough.” —Male, UK | “Sometimes when I…get…really sick…it feels like…an elephant sits on your chest…it hurts to breathe in.” —Male, North America | “…when she’s…coughing and…gets out of breath…and she’s quite pale…people do look. You do see them looking and thinking…what’s wrong with that child?” —Parent, UK |
| “…if you're out of breath it’s harder to breathe and that kind of frustrates you because there's nothing you can do half the time.” —Male, North America | |||
| Sinus symptoms | “…when I do have the pain it kind of stings and feels like somebody’s pushing down on my sinuses.” —Female, North America | “I feel like I’m being judged by other people because I constantly sniff and…cough.” —Female, UK | “she’s always blowing her nose and that becomes an issue… and she does worry.” —Parent, UK |
| Social functioning | “It’s a weird feeling because no one else has got it…you’re the only one that’s got it.” —Male, UK | “…if [my brother] goes [to] movies or even out with friends, anywhere, I might not be able to go because [of] my allergies.” —Male, North America | “She’s spent a vast amount of time in hospital, away from everybody else. She started school three months after everybody else... she does feel kind of segregated from everybody else.” —Parent, UK |
| “…sometimes I raise my hand and then say, ‘I have to blow my nose.’ And then I go in the bathroom…and shut the door because I don’t want anyone to hear me [because] it’s embarrassing.” —Female, North America | |||
| School functioning | “…if I can’t get out of bed because I’m hurting so bad, we don’t bother about bringing me to school. For first and second grade…a teacher from school would come to the house and…teach me…whenever I’m in the hospital… the teachers will…bring me my work.” —Female, North America | “All the teachers know about it and they try to help as best as they can….If they see that I’m struggling with anything, maybe with my hearing, because my hearing is quite bad as well…they try and explain to me a little bit louder and a little bit clearer.” —Female, UK | “She wears hearing aids. In her classroom even with her hearing aids, they have a speaker system for her and…[when] she was younger, she didn’t really mind, but now that she’s getting older, it makes her feel different.” —Parent, North America |
Definition of abbreviation: PCD = primary ciliary dyskinesia.
The UK relevance postal survey consisted of a list of 78 potential items for inclusion and was scored for relevance by 24 children, 9 teenagers, and 24 parent-proxies.
Item Generation: Content Analysis of Patient/Parent Interview Data and Relevance Survey
Content analysis of the transcripts yielded key items for each of 12 HRQoL domains, based on the frequency with which they were mentioned across respondents and the severity of their impact. Saturation of content across domains was confirmed when no new themes emerged (see Table 3 for an example saturation grid for adolescents). Overall, we achieved saturation of content by the third to twentieth interview, depending on the respondent and specific content area. Results of the relevance survey (Appendices E2–E4) overlapped closely with the issues discussed during open-ended interviews and the frequency distributions identified in the saturation matrices.
Table 3.
Saturation grids from open-ended interviews for North American and UK adolescents with primary ciliary dyskinesia: sinus symptoms and treatment burden
| Adolescents from North America and the UK |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | Total | |
| Sinus symptoms | |||||||||||||||||||||
| Runny nose | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
| Stuffy nose (congestion) | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 13 |
| Sinus headache | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 10 |
| Postnasal drip | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Facial pain | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Sinus infection | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Sore throat | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Treatment burden | |||||||||||||||||||||
| Hard to fit in treatments | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 12 |
| Prefer other activities to treatment | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 7 |
| Treatments are bothersome | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Don't want to do treatments | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 7 |
| Social barriers to treatment | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Difficulty remembering treatments | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Don't think treatments are necessary | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Treatments affect social life and other activities | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 |
Note: Participant endorsement of row item indicated by “1.” Boldface “1” indicates first endorsement of item by any participant. Saturation was reached by the end of the ninth interview for sinus symptoms and by the end of the third interview for treatment burden.
Development of Prototype Questionnaire
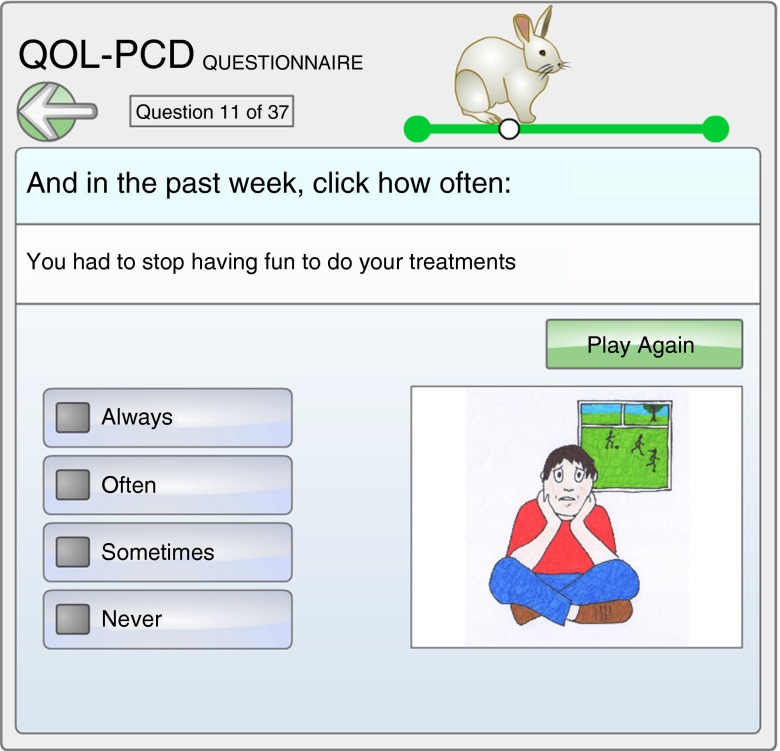
The first draft measure (QOL-PCD version 1.1) contained the following: (1) child version (9 scales, 43 items), (2) parent proxy version (10 scales, 50 items), and (3) adolescent version (11 scales, 52 items). We subjected all three measures to the Flesch–Kincaid Readability Index with the following results: (1) the child measure for those 6–12 years of age was at the fourth grade level (ages 9–10); to increase the readability and response effort for young children, we developed a pictorial version of the items, presented and “read” to the child by computer (Figure 2); (2) the adolescent version (13–17 yr) had a readability index of sixth grade (6.6), which is about 11 years; and (3) the parent version was at a 6.5 grade reading level.
Figure 2.
Image from the electronic children’s version of the QOL-PCD, illustrating the question “During this past week, indicate how often you had to stop having fun to do your treatments.” (Questionnaire printed with permissions, and image provided by Robert Scott.)
Cognitive Testing of Prototype Questionnaire
Cognitive interviews were conducted with 14 children, 16 adolescents, and 17 parents (Table 2). Review of these transcripts indicated that all respondents found the items clear and comprehensive, with rating scale options that were easy to use. However, some items were not viewed as relevant or important (e.g., “you thought you were too thin”; Body Image). These items were removed from each prototype questionnaire, based on patient/parent input after the cognitive testing phase (Table 4). Specifically, six items were removed from the child questionnaire; nine items were removed from the parent proxy questionnaire; and nine items were removed from the adolescent questionnaire.
Table 4.
Cognitive testing results for quality of life-primary ciliary dyskinesia questionnaires: final items and scales
| Child |
Adolescent |
Parent Proxy |
||||
|---|---|---|---|---|---|---|
| Scales | Items | Scales | Items | Scales | Items | |
| Version 1.1 | 9 | 43 | 11 | 52 | 10 | 50 |
| Version 1.2* | 7 | 37 | 9 | 43 | 9 | 41 |
| Deleted items | Vitality | You had trouble falling asleep. | Emotional Functioning | You felt frustrated about having PCD. | Physical Functioning | Carrying or lifting heavy objections such as books, a school bag, or backpack. |
| Eating and Weight | You had trouble eating. | Ears and Hearing | You felt pressure in your ears. | Emotional Functioning | Seemed short-tempered. | |
| Body Image | • You thought you were too short. | Respiratory Symptoms | You had tightness in your chest. | Ears and Hearing | My child had pressure in his/her ears. | |
| • You thought you were too thin. | Social Functioning | I feel lonely. | Respiratory Symptoms | My child had chest tightness. | ||
| • You thought you looked different from others your age. | • How do you feel about eating? | Sinus Symptoms | My child woke up during the night because his/her nose was blocked up. | |||
| Eating and Weight | • I have to push myself to eat. | Social Functioning | My child tends to be withdrawn. | |||
| • You had a poor appetite. | Eating and Weight | My child has less fun than usual. | ||||
| • Have you had trouble gaining weight? | Body Image | My child has trouble concentrating. | ||||
| Body Image | • I think I look different from others my age. | My child has had trouble gaining weight. | ||||
| • I feel bad about my physical appearance. | My child feels small compared to other kids the same age. | |||||
| • I think I am smaller than others my age. | My child feels physically different from other kids the same age. | |||||
| My child thinks that he/she is too thin. | ||||||
| Deleted scales | • Eating and Weight | • Eating and Weight | • Body Image | |||
| • Body Image | • Body Image | |||||
Definition of abbreviation: PCD = primary ciliary dyskinesia.
Version 1.2 developed after cognitive testing of version 1.1 and now being validated.
Because of lack of endorsement, items assessing domains such as body image, eating and weight were removed from the child and adolescent instruments (e.g., “I think I look different from others my age,” “I think I am smaller than others my age,” “I have to push myself to eat,” and “Have you had trouble gaining weight?”). Thus, the final prototype instruments (QOL-PCD version 1.2) contained the following numbers of items: (1) child version (37 items), (2) parent proxy version (41 items), and (3) adolescent version (43 items) (Table 5).
Table 5.
Quality of life-primary ciliary dyskinesia scales in the three age-appropriate instruments*
| Child (ages 6–12 yr) | Adolescent (ages 13–17 yr) | Parent Proxy (for children 6–12 yr) | |
|---|---|---|---|
| Physical Functioning | You were able to climb stairs as fast as others | You had difficulty performing activities such as running or playing sports | Your child had difficulty performing vigorous activities, such as running or playing sports |
| Emotional Functioning | You were teased by other children because your nose was runny | You felt worried about getting sick | Your child seemed worried about his/her illness |
| Treatment Burden | Doing your treatments bothered you | Your treatments for PCD got in the way of your activities | Your child was frustrated by doing his/her treatments |
| Ears and Hearing | Your ears hurt | You had trouble hearing (if you wear hearing aids: you had trouble hearing without your aids) | My child had fluid draining from his/her ears |
| Respiratory Symptoms | You had to cough up mucus (even if you swallow it) | You had difficulty sleeping because of your chest | My child woke up during the night because he/she was coughing |
| Sinus Symptoms | You woke up at night because your nose was blocked up | You had difficulty sleeping because your nose was blocked up | My child had a runny nose |
| Social Functioning | You missed going to after-school activities because of your PCD | I think my coughing bothers others | NA |
| Role Functioning | NA | It is difficult to make plans for the future (e.g., going on in school, getting a job, etc.) | NA |
| Vitality | NA | You felt exhausted | My child was able to keep up with his/her school work or outdoor activities |
| Health Perceptions | NA | NA | My child led a normal life |
| School Functioning | NA | NA | My child got enough help in his/her classroom to perform well (e.g., sitting up front…) |
| Eating and Weight | NA | NA | Mealtimes were a struggle |
Definition of abbreviations: NA = scale not applicable for the QOL-PCD instrument for this respondent type; PCD = primary ciliary dyskinesia.
Due to copyright and need for further psychometric validation before widespread use, only a sample of items are provided for the reader (one example item reported per scale per instrument). Interested readers should contact the authors if they wish to use these instruments. Items refer to a 1-week recall period and responses are rated on a four-point Likert scale. Respondents are instructed to answer questions from their own perception.
Discussion
The primary goal of this study was to develop the first HRQoL instruments for pediatric patients with PCD and parent caregivers, following guidelines on the development of patient-reported outcome measures established by the FDA and EMA. Using data from both North America and the United Kingdom, we conducted an expert clinician panel, focus groups, and individual open-ended interviews, followed by item generation and cognitive testing. This process yielded separate instruments for school-age children, adolescents, and parents. Similar processes were used to develop an HRQoL measure for adults with PCD (22). These instruments can be used to document the progression of disease, monitor patients clinically, and serve as an outcome measure for clinical trials on the impact of new therapies from the patient perspective. In PCD, this is particularly important given that there are few physiologic end points that can be used for these purposes.
The key principle governing all phases of instrument development was our inclusion of patient and parent input at each phase. To ensure the generalizability and validity of these measures, we developed them cross-culturally in English-speaking countries (Canada, the United States, the United Kingdom, Ireland) and found few differences in critical item content across countries, despite using slightly different methodology for questionnaire development in North America and Europe. All phases of instrument development required by relevant regulatory bodies were followed in North America and Europe. Additional input was obtained from published literature and medical experts in these locations. These processes have yielded reliable and valid HRQoL instruments for a variety of chronic conditions, such as CF and bronchiectasis (23, 24).
Open-ended and cognitive interviews highlighted the importance of the impact of respiratory and sinus symptoms on patients’ daily functioning. These symptoms were similar to those reported by patients with CF and bronchiectasis (12, 25) and included chronic cough, mucus production, trouble breathing, and sinus headaches. However, several items that appear on the final QOL-PCD measures reflect symptoms and functioning that are unique to PCD, such as runny nose, congestion in the nose, chronic otitis media, difficulty hearing, speech delays, and in some cases, a need for special accommodations in the classroom. These distinct symptoms are attributable to the abnormal functioning of the cilia, which disrupts normal mucus clearance from the lungs, nose, sinuses, and middle ear (1). In contrast, CF is characterized by predominantly lower respiratory tract and gastrointestinal symptoms, and ear disease is not a feature of CF.
Furthermore, unlike individuals with CF, patients with PCD did not endorse symptoms related to body image, gastrointestinal discomfort, or eating and weight problems. Patients with PCD are pancreatic sufficient, and although their appetite may decrease when they are ill (i.e., during a pulmonary exacerbation), they do not have the primary nutritional and growth issues associated with CF. Hence, this finding provides additional support for the content validity of our measures.
Importantly, this study demonstrated differences in HRQoL based on respondent age. Younger children did not mention more abstract concepts, such as health perceptions, vitality, and role functioning, which is typical across disease-specific patient-reported outcomes (22). In contrast, adolescents reported that PCD affected their energy levels and functioning at school. These findings underscore the importance of using developmentally appropriate HRQoL instruments that reflect the respondent’s cognitive and developmental stage, and that will optimize sensitivity and specificity (15, 23, 26). It is also advantageous to have both child and parent proxy versions of these measures for younger children to facilitate comparisons between parent–child dyads, and to identify a fuller picture of the effects of PCD on daily functioning, allowing us to evaluate children’s HRQoL from the earliest possible age (26).
Strengths and Limitations
A strength of this study was our success in recruiting a large and geographically disparate sample of patients with this rare disease from North America and Europe. These samples represented different ethnic groups, including Pakistani and Irish travelers (a European minority group). However, because participants were required to understand and speak English, we likely oversampled white participants. Although we did not have African Americans in our samples, to date, PCD has been described in only a few patients from this race (17).
A limitation of our study is that we used convenience sampling methods, but importantly, we used a wide range of recruiting strategies, including advertisements in patient advocacy newsletters, national PCD education days, and patients seen in clinic settings. This enabled us to include a sociodemographically diverse patient population. In the next phase of instrument development, national psychometric testing, we will systematically evaluate the generalizability of these instruments.
Summary
QOL-PCD measures have now been developed using the most recent guidance from the FDA and EMA, and have undergone cognitive testing in pediatric patients from several English-speaking countries. Despite the rarity of this disease, we used two key collaborative projects (GDMCC [National Institutes of Health, Bethesda, MD] and European Union BESTCILIA) to recruit a substantial number of patients with a confirmed diagnosis of PCD.
These instruments have already been translated into Dutch, German, Danish, French, and Greek with plans to develop additional translations for other North American populations (Latin American Spanish) and major countries in Europe and the Middle East. A multinational, psychometric field study is underway to assess item and scale reliability, convergent and divergent validity, and responsiveness. These questionnaires are expected to be useful as end points in clinical trials, for monitoring health outcomes in prognostic studies, for generating quality improvement initiatives, and for improving clinical decision-making.
Footnotes
Supported by grants to M.R.K., S.D.D., T.W.F., and M.W.L.: U54HL096458 from the National Institutes of Health (NIH) through the Genetic Disorders of Mucociliary Clearance Consortium (GDMCC), an initiative of the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the National Heart, Lung, and Blood Institute (NHLBI); A.L.Q. was supported by an investigator-initiated grant, Gilead Sciences; S.D.D. received grant funding from Maya’s March, SickKids Foundation; J.S.L., A.L.Q., and M.W.L. received grant funding from the European Union’s Seventh Framework Program under EC-GA No. 305404 BESTCILIA. The National PCD Centres in Southampton and London are commissioned and funded by NHS England. Research in Southampton is supported by the NIHR Southampton Respiratory Biomedical Research Unit, NIHR Wellcome Trust Clinical Research Facility, and AAIR Charity. Members of the PCD European Group provided expert opinion; J.S.L., M.W.L., C.H., and L.B. are members of the ERS Task Force for PCD Diagnostics (ERS TF-2014-04) and EU-funded COST Action BEAT-PCD (BM1407). Members of the PCD Foundation, North America (Director, Michele Manion) and PCD Support Group, UK (Chair, Fiona Copeland) contributed to all aspects of study conduct.
Prior abstract publication/presentation: Dell S, Leigh M, Ferkol T, Knowles M, Alpern A, Behan L, Hogg C, DunnGalvin A, Lucas JS, Quittner AL. Development of the QOL-PCD: cross-cultural pediatric patient–reported outcome measures for global clinical trials. European Respiratory Society Congress, Munich, September 2014.
Author Contributions: S.D.D., J.S.L., M.W.L., and A.L.Q. guarantee the integrity of the work from inception to the published article. S.D.D., J.S.L., M.W.L., and A.L.Q. conceived and designed the study. S.D.D., J.S.L., M.W.L., M.R.K., T.W.F., and C.H. recruited patients. A.A., L.B., A.M.M., and A.L.Q. conducted interviews. A.L.Q. and J.S.L. conducted qualitative analyses. J.S.L., M.W.L., L.B., A.D., and A.L.Q. participated in teleconferences to develop and modify prototypes and the final version of QOL-PCD. S.D.D. and A.L.Q. wrote the manuscript. All authors contributed to the study design, interpretation of data, and manuscript writing.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia: recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro AJ, Zariwala MA, Ferkol T, Davis SD, Sagel SD, Dell SD, Rosenfeld M, Olivier KN, Milla C, Daniel SJ, et al. Genetic Disorders of Mucociliary Clearance Consortium. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol. 2016;51:115–132. doi: 10.1002/ppul.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quittner AL, Modi A, Cruz I. Systematic review of health-related quality of life measures for children with respiratory conditions. Paediatr Respir Rev. 2008;9:220–232. doi: 10.1016/j.prrv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Maglione M, Bush A, Montella S, Mollica C, Manna A, Esposito A, Santamaria F. Progression of lung disease in primary ciliary dyskinesia: is spirometry less accurate than CT? Pediatr Pulmonol. 2012;47:498–504. doi: 10.1002/ppul.21569. [DOI] [PubMed] [Google Scholar]
- 5.Green K, Buchvald FF, Marthin JK, Hanel B, Gustafsson PM, Nielsen KG. Ventilation inhomogeneity in children with primary ciliary dyskinesia. Thorax. 2012;67:49–53. doi: 10.1136/thoraxjnl-2011-200726. [DOI] [PubMed] [Google Scholar]
- 6.Irving SJ, Ives A, Davies G, Donovan J, Edey AJ, Gill SS, Nair A, Saunders C, Wijesekera NT, Alton EW, et al. Lung clearance index and high-resolution computed tomography scores in primary ciliary dyskinesia. Am J Respir Crit Care Med. 2013;188:545–549. doi: 10.1164/rccm.201304-0800OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon M, Vermeulen FL, Gysemans W, Proesmans M, Jorissen M, De Boeck K. Lung structure–function correlation in patients with primary ciliary dyskinesia. Thorax. 2015;70:339–345. doi: 10.1136/thoraxjnl-2014-206578. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services Food and Drug Administration Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims 2009. Dec [updated 2009 Dec; accessed 2015 September 24]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf
- 9.Apolone G, De Carli G, Brunetti M, Garattini S. Health-related quality of life (HR-QOL) and regulatory issues: an assessment of the European Agency for the Evaluation of Medicinal Products (EMEA) recommendations on the use of HR-QOL measures in drug approval. Pharmacoeconomics. 2001;19:187–195. doi: 10.2165/00019053-200119020-00005. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency Committee for Medicinal Products for Human Use (CMHP). Reflection paper on the regulatory guidance for the use of health related quality of life (HRQL) measures in the evaluation of medicinal products 2005[accessed 2015 August 4]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_001238.jsp&mid=WC0b01ac0580032ec4
- 11.Willke RJ, Burke LB, Erickson P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Control Clin Trials. 2004;25:535–552. doi: 10.1016/j.cct.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of the Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128:2347–2354. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 13.Goss CH, Quittner AL. Patient-reported outcomes in cystic fibrosis. Proc Am Thorac Soc. 2007;4:378–386. doi: 10.1513/pats.200703-039BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:921–928. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landgraff J, Abetz L. Measuring health outcomes in pediatric populations: issues in psychometrics and application. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. Philadelphia: Lippincott-Raven; 1996. pp. 793–802. [Google Scholar]
- 16.Dell S, Leigh M, Ferkol T, Knowles M, Alpern A, Behan L, Hogg C, DunnGalvin A, Lucas JS, Quittner AL.Development of pediatric cross-cultural patient-reported outcome measures: QOL-PCD [abstract]. Presented at the European Respiratory Society Annual Congress, September 2014. 44 [Google Scholar]
- 17.Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, Sagel SD, Milla C, Zariwala MA, Pittman JE, Shapiro AJ, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015;191:316–324. doi: 10.1164/rccm.201409-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, Bartoloni L, Eber E, Escribano A, Haarman E, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly M, Parker N. “Unsatisfactory saturation”: a critical exploration of the notion of saturated sample sizes in qualitative research. Qual Res. 2013;13:190–197. [Google Scholar]
- 20.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311:376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty PC, Willis GB. Research synthesis: the practice of cognitive interviewing. Public Opin Q. 2007;71:287–311. [Google Scholar]
- 22.Lucas JS, Behan L, Dunn Galvin A, Alpern A, Morris AM, Carroll MP, Knowles MR, Leigh MW, Quittner AL. A quality-of-life measure for adults with primary ciliary dyskinesia: QOL-PCD. Eur Respir J. 2015;46:375–383. doi: 10.1183/09031936.00216214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quittner AL, Sawicki GS, McMullen A, Rasouliyan L, Pasta DJ, Yegin A, Konstan MW. Psychometric evaluation of the Cystic Fibrosis Questionnaire-Revised in a national sample. Qual Life Res. 2012;21:1267–1278. doi: 10.1007/s11136-011-0036-z. [DOI] [PubMed] [Google Scholar]
- 24.Quittner AL, O’Donnell AE, Salathe MA, Lewis SA, Li X, Montgomery AB, O’Riordan TG, Barker AF. Quality of Life Questionnaire-Bronchiectasis: final psychometric analyses and determination of minimal important difference scores. Thorax. 2015;70:12–20. doi: 10.1136/thoraxjnl-2014-205918. [DOI] [PubMed] [Google Scholar]
- 25.Quittner AL, Marciel KK, Salathe MA, O’Donnell AE, Gotfried MH, Ilowite JS, Metersky ML, Flume PA, Lewis SA, McKevitt M, et al. A preliminary Quality of Life Questionnaire-Bronchiectasis: a patient-reported outcome measure for bronchiectasis. Chest. 2014;146:437–448. doi: 10.1378/chest.13-1891. [DOI] [PubMed] [Google Scholar]
- 26.Alpern AN, Brumback LC, Ratjen F, Rosenfeld M, Davis SD, Quittner AL. Initial evaluation of the Parent Cystic Fibrosis Questionnaire—Revised (CFQ-R) in infants and young children. J Cyst Fibros. 2015;14:403–411. doi: 10.1016/j.jcf.2014.11.002. [DOI] [PubMed] [Google Scholar]