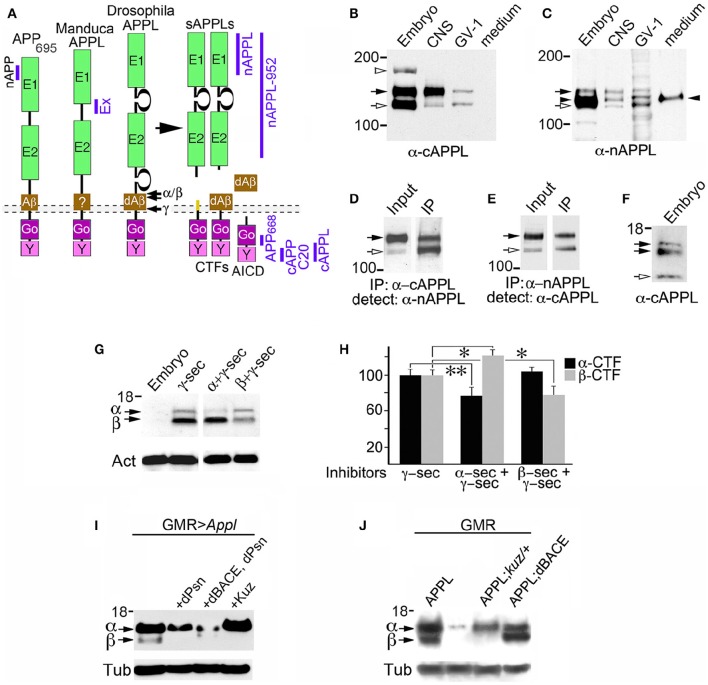
Figure 1.
Insect APPL is cleaved by the same secretase classes that process APP. (A) Schematic image of the primary domains shared by human APP695 and APPL in Drosophila and Manduca. All APP family members contain similar extracellular domains (E1 and E2) that can interact with potential binding partners; a highly conserved cytoplasmic domain (Go) that directly interacts with the heterotrimeric G protein Goα; and a C-terminal tyrosine-based sorting motif (Y) that interacts with a variety of intracellular adapter and signaling molecules. Drosophila APPL contains larger non-conserved regions on either side of the E2 domain that increase the overall size of the holoprotein, and an Aβ-like domain (dAβ) with neurotoxic activity when cleaved from the holoprotein; the biological activity of this domain in Manduca APPL has not yet been verified. Similar to the cleavage products of APP695, processing of insect APPL by α- and β-secretases produces soluble ectodomain fragments (sAPPLs) and short transmembrane C-terminal fragments (CTFs); subsequent cleavage of the CTFs by γ-secretase produces an APPL intracellular domain (AICD), as well the dAβ peptide or a p3-like fragment (not shown). Labeled blue bars indicate the epitopes recognized by antibodies against APPL or APP that were used in this study (as described in the Materials and Methods Section). (B,C) Western blots of lysates prepared from Manduca embryos (65 HPF), 5th instar CNS, Manduca GV-1 cells (which endogenously express APPL), and concentrated medium harvested from the GV-1 cultures. (B) Immunoblotting with anti-cAPPL detects both the mature (black arrow) and immature (open arrow) full-length forms of APPL in all three lysates but not in GV-1 cell medium; a larger band (~165 kDa; open arrowhead) detected in mid-stage embryos might represent an additional post-translational modification that is developmentally regulated (as previously reported; Swanson et al., 2005). (C) Immunoblotting with anti-nAPPL detects the same mature (black arrow) and immature (open arrow) full-length forms of APPL, plus cleaved ectodomain fragments (sAPPLs) that are also present in GV-1 medium (black arrowhead). The relative intensity of this ectodomain band reflects the rapid processing of full-length APPL; sAPPL produced by α- vs. β-secretases were not distinguished in this blot. (D,E) Cross-immunoprecipitation of Manduca embryonic lysates with N- and C-terminal-specific antibodies against APPL. (D) Embryonic lysate (input) that was immunoprecipitated with anti-cAPPL (IP) and immunoblotted with anti-nAPPL. (E) Embryonic lysate (input) that was immunoprecipitated with anti-nAPPL (IP) and immunoblotted with anti-cAPPL; both antibodies recognize mature (black arrow) and immature (open arrow) forms of full-length APPL. (F) Western blot of Manduca embryo lysate (lower portion) labeled with anti-cAPPL reveals two CTFs (black arrows) and a candidate AICD fragment (open arrowhead). (G) Western blot of Manduca embryo lysates treated with different secretase inhibitors; in this shorter exposure (compared to F), neither CTF was detected (black arrows). In lysates of embryos treated with a γ–secretase inhibitor (lane 2), both CTFs were readily detected. Treatment with a combination of α- plus γ-secretase inhibitors reduced the relative abundance of the upper CTF band, whereas treatment with β- plus γ-secretase inhibitors reduced the lower CTF band. Separate band labeled with “Act” indicates anti-actin (~42 kDa) as a loading control. (H) Quantification of CTF abundance in western blots of embryonic lysates (as illustrated in G). Treatment with α- plus γ-secretase inhibitors caused a significant decrease in α-CTF levels (**p = 0.0002) and a more moderate increase in β-CTF levels (*p = 0.041). Treatment with β- plus γ-secretase inhibitors caused a significant reduction in β-CTF (*p = 0.041) but did not affect α-CTF levels (p = 0.101). Relative intensities were normalized against γ-secretase-treated lysates in each immunoblot. N ≥ 10 for each group; histograms show means ± SEM. Statistical comparisons were performed using one-way ANOVA followed by pairwise Student's two-tailed t-tests with the Bonferroni correction to obtain reported p-values. (I) Western blots of head lysates from flies expressing additional APPL in the eye (GMR-GAL4; UAS-Appl), immunoblotted with anti-cAPPL. Lane 1, both α- and β-CTFs (arrows) could be readily detected in GMR>Appl flies. Lane 2, expressing additional Drosophila Presenilin in this line (via UAS-dPsn) reduced α- and β-CTFs (β-CTF was no longer detectable at this exposure). Lane 3, expressing additional dPsn plus Drosophila BACE (via UAS-dPsn + UAS-dBACE) preferentially reduced α-CTF levels (β-CTF was still detectable, compared to lane 2). Lane 4, expressing additional Kuzbanian in this line (via UAS-Kuz) caused a marked increase in α-CTF and a corresponding reduction in β-CTF levels. (J) Western blots of head lysates from flies carrying the eye-specific promoter construct GAL4-GMR, immunoblotted with anti-cAPPL. Lane 1, in flies overexpressing APPL (via UAS-Appl), both α- and β-CTFs (arrows) could be readily detected (as in panel I, lane 1). Lane 2, in GMR-GAL4 control flies, only the α-CTF band was faintly detected at this exposure. Lane 3, both CTFs were reduced in flies lacking one copy of the α-secretase Kuzbanian (kuz/+). Lane 4, co-expressing additional APPL and dBACE caused a preferential increase in β-CTF levels. Separate bands in (I,J) labeled with “Tub” show anti-tubulin (~55 kDa) as a loading control.