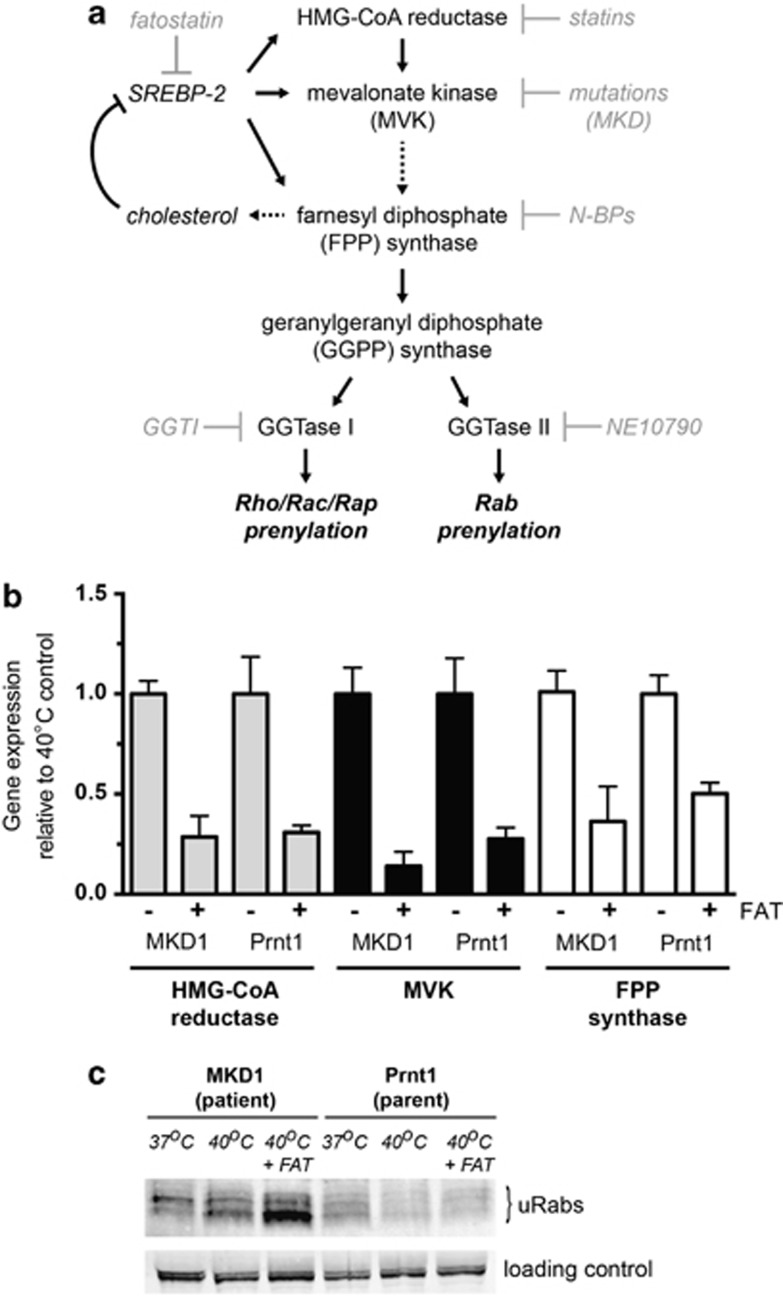
Figure 2.
Fatostatin (FAT) decreases SREBP-dependent gene expression and enhances the inhibition of protein prenylation at 40 °C in MKD cells. (a) Schematic diagram of the flux through the mevalonate pathway leading to sterol biosynthesis and protein prenylation. Mutations resulting in reduced MVK activity, as well as pharmacological inhibitors such as statins and nitrogen-containing bisphosphonates (N-BPs), block the production of isoprenoid precursors essential for protein prenylation. The specific inhibitors GGTI-298 and NE10790, respectively, inhibit prenylation of Rho/Rac/Rap GTPases and Rab GTPases. FAT interferes with the upregulation of genes encoding mevalonate pathway enzymes by blocking SREBP-2 activation. (b) TaqMan gene expression analysis of three SREBP-regulated genes in patient (MKD1) and parent (Prnt1) EBV-LCLs shows substantial reduction upon FAT treatment. Cells were cultured with dimethyl sulfoxide vehicle (−) or 10 μM FAT (+) at 40 °C for 2 days before harvesting for RNA isolation and real time PCR. dd-CT values are the mean of three separate experiments and expressed relative to the vehicle control (± s.e.m.). (c) FAT increased the accumulation of unprenylated Rab proteins (uRabs) that occurs in MKD1 (patient) cells after culture for 3 days at 40 °C, but has no effect in Prnt1 (parent) cells. Endogenous 75 kDa biotinylated protein was used as a loading control. The data shown are representative of three independent experiments.