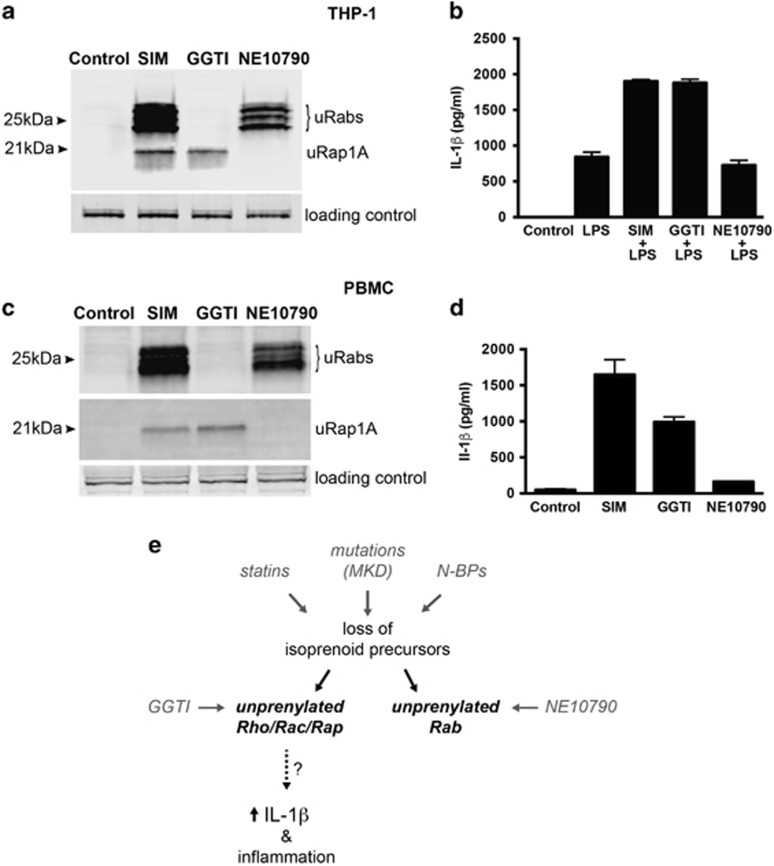
Figure 3.
Inhibition of Rab prenylation by NE10790 does not enhance IL-1β release from THP-1 monocytes. Unprenylated Rab GTPases (uRabs) or unprenylated Rap1A (uRap1A) were detected in THP-1 lysates (a) or human PBMC lysates (c) after treatment for 24 h with 5 μM simvastatin (SIM), 10 μM GGTI-298 or 2 mM NE10790. Endogenous 75 kDa biotinylated protein was used as a loading control. The data shown are representative of three independent experiments. (b) Pretreatment of THP-1 cells for 24 h with 5 μM SIM or 10 μM GGTI-298 enhanced the release of mature IL-1β after stimulation with 200 ng ml−1 LPS for 6 h, whereas pretreatment with 2 mM NE10790 had no effect. (d) Treatment of human PBMC for 24 h with 5 μM SIM or 10 μM GGTI-298 caused the release of mature IL-1β, whereas 2 mM NE10790 had no effect. ELISA values in b, d are the mean ± s.d. of triplicate samples and are representative of at least two independent experiments. (e) Disruption of the mevalonate pathway, by pharmacological inhibition or mutations in MVK, results in reduced synthesis of isoprenoid lipid precursors essential for protein prenylation. However, it is the loss of prenylation of GTPases modified by GGTase I (e.g., Rho, Rac and Rap), and not the loss of prenylated Rab GTPases (prenylated by GGTase II), that causes excessive IL-1-β production.