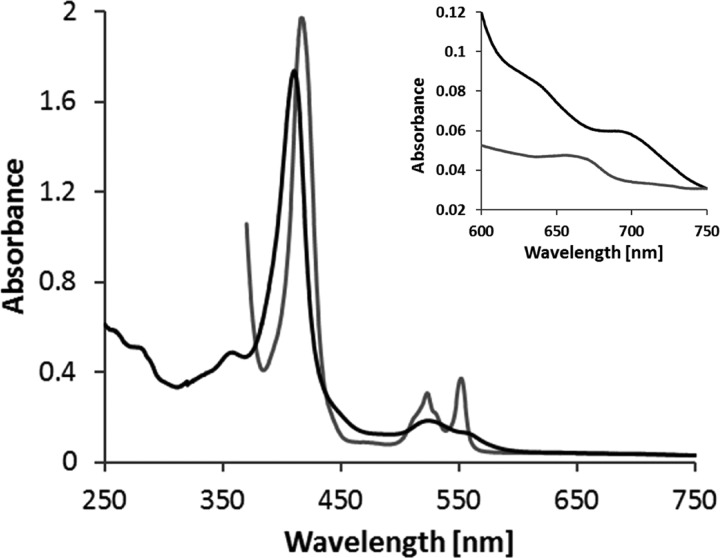
FIGURE 2.
UV-visible spectra of TsdB from S. lithotrophicus. As the protein is partly reduced in the “as isolated” state, up to 170 μm ferricyanide were added to record the oxidized spectrum (black line). For full reduction of the protein, sodium dithionite was added (gray line). 100 mm Tris buffer, pH 8.0, with 150 mm NaCl and 2.5 mm desthiobiotin was used, and spectra are normalized to 750 nm. The oxidized spectrum exhibits a 700-nm peak indicating methionine as heme iron ligand. Protein concentration was 6 μm in the overview and 29 μm in the blowup.