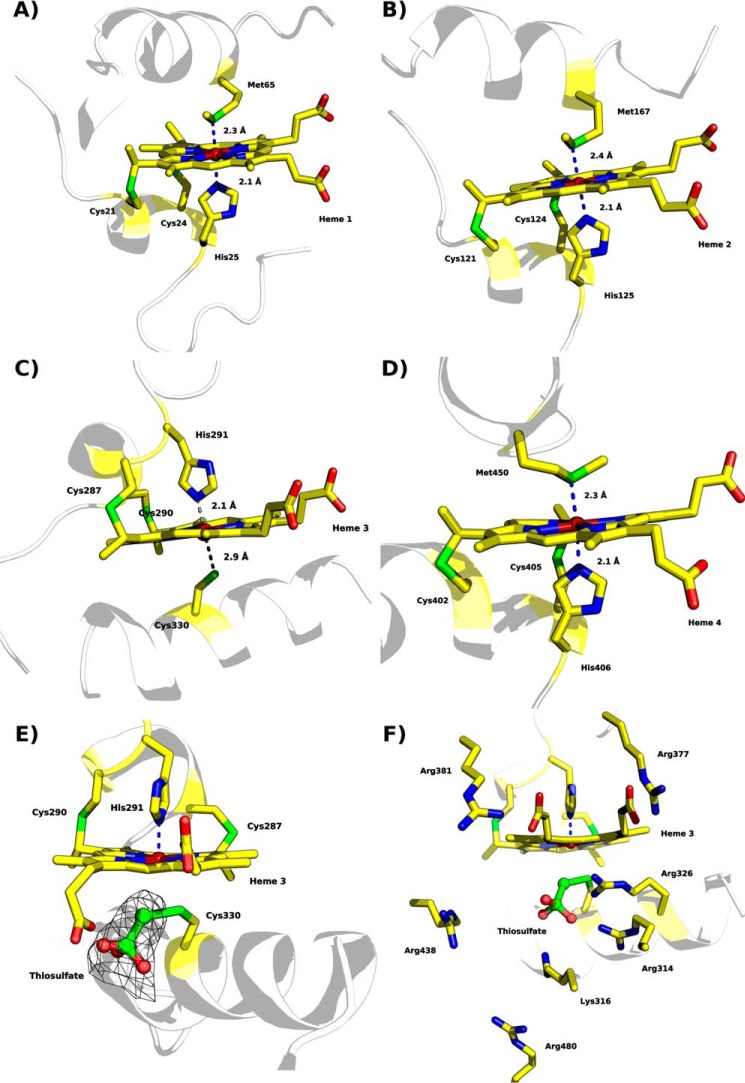
FIGURE 8.
Heme coordination of “as isolated” MpTsdBA (PDB code 5LO9). A, heme 1 is coordinated by His25 and Met65. B, heme 2 is coordinated by His125 and Met167. C, heme 3 is ligated to His291 but not to Cys330. The distance of Sγ to the heme iron is 2.9 Å and thus not close enough for direct ligation. Thiosulfate covalently bound to Sγ of Cys330 is not shown here for clarity. Presence of thiosulfate is illustrated in detail in E and F. D, Heme 4 is ligated by His406 and Met450. E, heme 3 with Sγ of Cys330 covalently bound to thiosulfate, displayed in ball and stick, and polder map electron density contoured at 6σ level depicted as a black mesh. F, heme 3 in a similar view as in E but with positively charged residues surrounding the substrate cleft depicted as sticks. Scheme representation is shown in pale gray with heme moieties and coordinating amino acid residues shown as sticks; color code as in Fig. 7 with sulfur atoms in green.