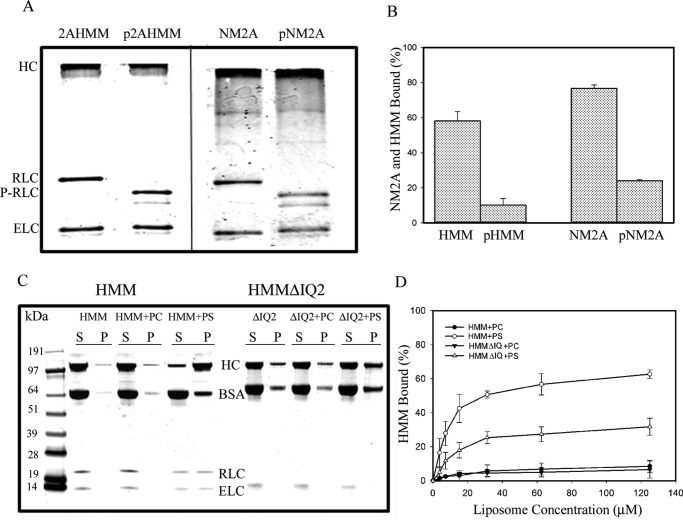
FIGURE 9.
Phosphorylation of myosin-bound RLC and deletion of RLC-binding site affects binding of NM2A-HMM and NM2A to 100% PS-liposomes. A, urea-glycerol gel electrophoretic analysis of phosphorylation of NM2A and NM2A-HMM by myosin light chain kinase. The RLC of both pHMM and pNM2A was fully phosphorylated (P-RLC). The upper pRLC band is monophosphorylated RLC, and the lower band is diphosphorylated RLC. B, RLC phosphorylation almost completely inhibited the binding of 100 nm NM2A and 100 nm NM2A-HMM to 30 μm PS-liposomes. C and D, deletion of the RLC-binding site (HMMΔIQ2) inhibited binding of HMM to PS-liposomes by ∼50%. C, SDS-PAGE of centrifugal supernatant and pellets of 100 nm HMM and HMMΔIQ2 incubated with 125 μm PC- or PS-liposomes. D, 100 nm HMM and HMMΔIQ2 incubated with 125 μm PC- or PS-liposomes. The data in B and D are the average and S.D. of at least three experiments.