FIGURE 2.
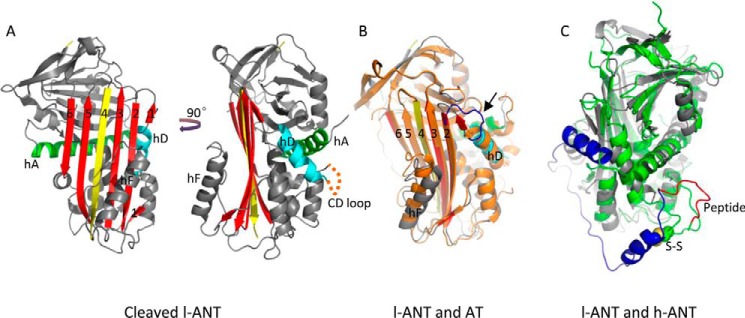
Overall structure of cleaved l-ANT. A, the structure of cleaved l-ANT exhibits a very typical serpin fold. The RCL (yellow) cleaved by human α-thrombin is completely inserted into central β-sheet A (red) as a middle strand. A seventh chain of β-sheet A (termed s1′A here) is seen above helix D (cyan), composed of residues 162–164. The N-terminal residues 1–72 and residues 138–147 between helix C and helix D (indicated with an orange dashed line) are not built into the structure because of poor electron density. Helix A is shown in green. B, overlaid structures of l-ANT and latent antithrombin (AT) (orange, PDB code 1AZX) indicate a large shift in helix F position and a different conformation of the connecting loop (blue) on top of helix D in antithrombin. C, overlaid structures of l-ANT (gray) and human angiotensinogen (h-ANT) (green, PDB code 2WXW). The N-terminal fragment of human angiotensinogen is shown in blue, and the hormone peptide is shown in red. The Cys-18–Cys-138 disulfide bond of human angiotensinogen is shown as spheres.