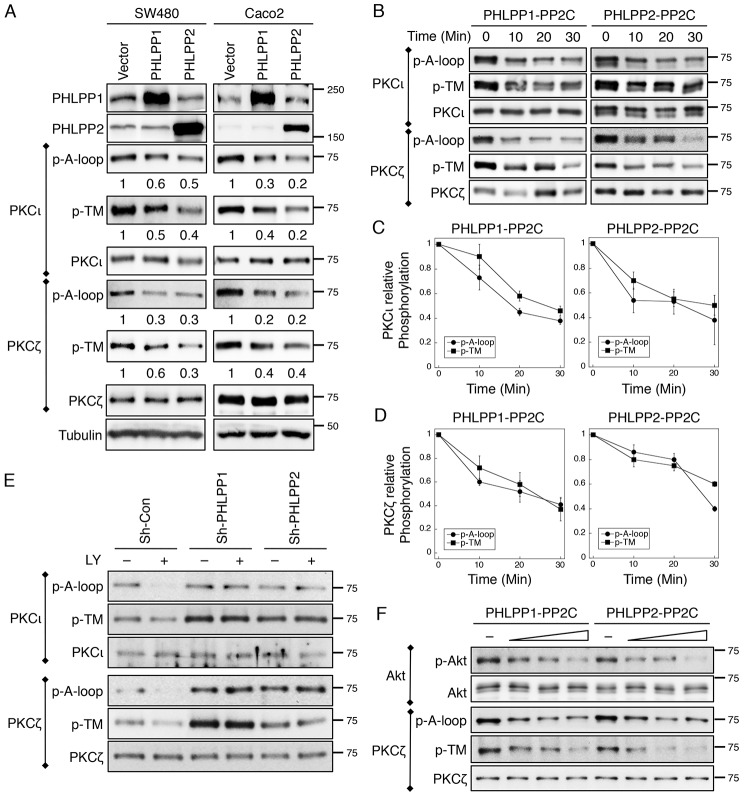
FIGURE 6.
PKCζ and PKCι are substrates of PHLPP. A, cell lysates prepared from SW480 and Caco2 cells stably overexpressing PHLPP1 or PHLPP2 were immunoprecipitated using antibodies against PKCζ and PKCι and subsequently analyzed by Western blotting. The phosphorylation status of PKCι and PKCζ at the A-loop and the TM site was detected using the phospho-specific antibodies. The relative phosphorylation was quantified by normalizing ECL signals generated by the phospho-specific antibody to that of total protein and is shown below the phosphoblots. B, the time course of aPKC dephosphorylation in vitro. 293T cells transfected with PKCζ and PKCι expression plasmids were lysed and immunoprecipitated using antibodies against PKCι or PKCζ. Dephosphorylation reactions were carried out by incubating the immunoprecipitates with the purified PP2C domains of PHLPP1 or PHLPP2 at room temperature for 0–30 min. The phosphorylation of aPKCs was detected using the phospho-antibodies. C and D, the levels of PKCι and PKCζ phosphorylation at the A-loop and TM sites were quantified by normalizing ECL signals generated by the phospho-specific antibodies to that of total protein. Data shown in the graph represent the means ± S.E. (n = 3). E, knockdown of PHLPP prevents dephosphorylation of PKCι and PKCζ. Stable sh-Con, sh-PHLPP1, and sh-PHLPP2 knockdown Caco2 cells were treated with LY294002 (LY, 20 μm) for 30 min. The phosphorylation status of immunoprecipitated PKCι and PKCζ was analyzed using the phospho-specific antibodies. F, the dose-dependent dephosphorylation of Akt and PKCζ in vitro. 293T cells transfected with Akt and PKCζ expression plasmids were lysed and immunoprecipitated using antibodies against Akt or PKCζ. Dephosphorylation reactions were carried out by incubating the immunoprecipitates with increasing amounts of PP2C domains of PHLPP1 or PHLPP2 at room temperature for 10 min. The phosphorylation of Akt and PKCζ was detected using the phospho-antibodies.