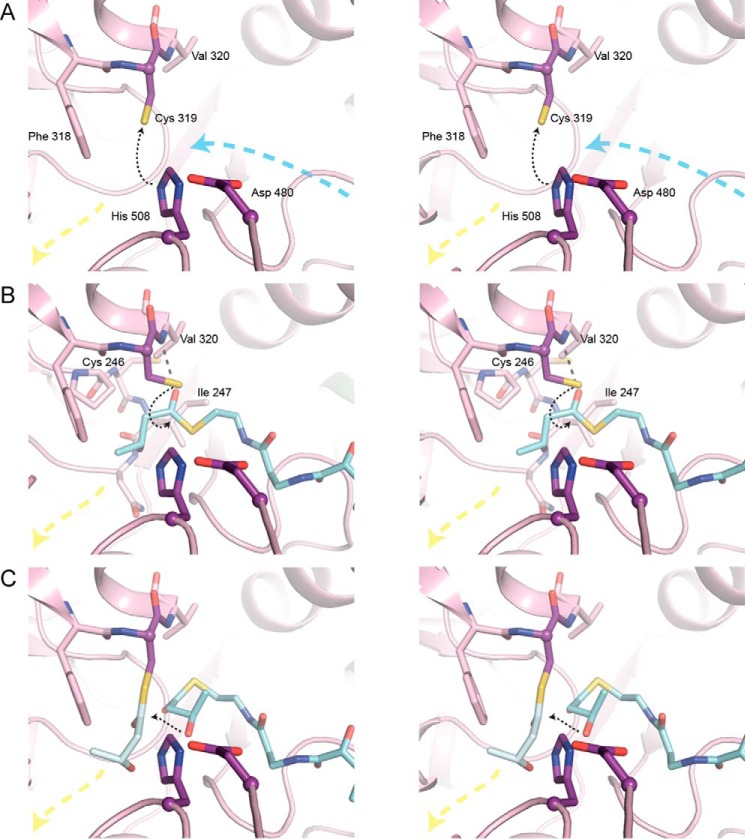
FIGURE 6.
Stereo views of HB-CoA modeled into the CnPhaC catalytic domain. A, active site of CnPhaC(C319A) in the absence of substrate. Black dashed arrow indicates deprotonation of Cys319 by His508. The position of the Cys319 sulfur atom is modeled. Blue and yellow dashed arrows represent the directions of the proposed substrate entrance and product egress channels, respectively. B, active site of CnPhaC(C319A) with HB-CoA (carbon atoms in cyan) modeled for initiation. Black dashed arrow represents path of nucleophilic attack of Cys319 on the HB-CoA thioester. The position of the sulfur atom of Cys319 is modeled. Gray dashed line represents a modeled hydrogen bond between the carbonyl oxygen of HB-CoA and the amide group of Val320. Cys246 and Ile247 are located on a loop just behind the nucleophile elbow. The amide group of one of these residues, in combination with the Val320 amide, could form the oxyanion hole to stabilize the tetrahedral intermediate during catalysis, although a conformational change within the active site upon substrate binding would be necessary to bring the loop close enough to form the appropriate interaction with the substrate carbonyl. C, active site of CnPhaC(C319A) with HB monomer (carbon atoms in gray) modeled onto Cys319 and HB-CoA (carbon atoms in cyan) modeled for polymer elongation. Black dashed arrow represents path of nucleophilic attack of the HB-CoA hydroxyl group on the protein-bound thioester.