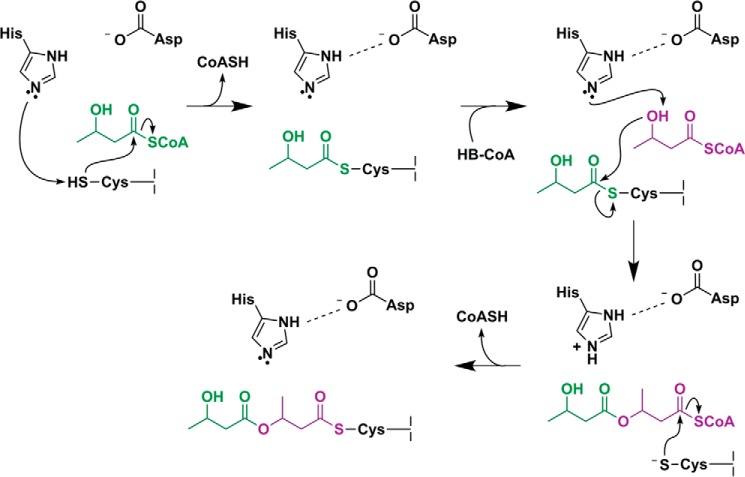
FIGURE 7.
Modified mechanistic scheme for PhaC. His508 deprotonates Cys319, allowing for nucleophilic attack on the HB-CoA thioester; the histidine base is regenerated by transfer of the proton to the CoASH leaving group. A second HB-CoA substrate binds, and the HB hydroxyl group is deprotonated by His508, facilitated through modulation of the histidine basicity by Asp480. The newly formed HB alkoxide attacks the Cys-HB thioester, generating a noncovalent, CoA-bound intermediate. The growing PHB chain is then transferred back to Cys319.