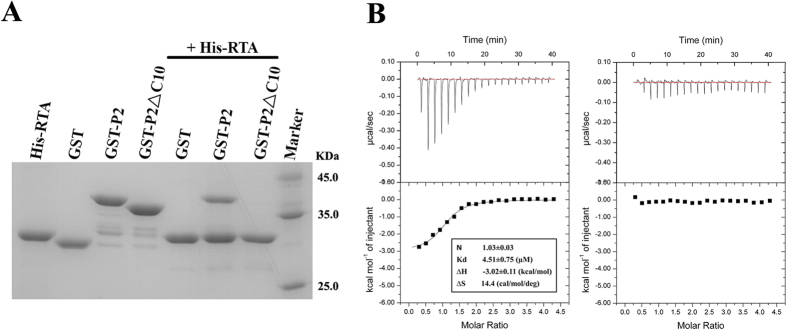
Figure 1. RTA interacts with the conserved C-terminal tail of P2.
(A) Pull-down analysis of the interaction between RTA and the C-terminal tail of P2. His-RTA bound to wild-type GST-P2 in vitro. GST-P2ΔC10 completely lost its ability to associate with His-RTA. His-RTA, GST, GST-P2 and GST-P2ΔC10 were used as controls. (B) ITC-based measurements of the binding affinity between RTA and wild-type P2 (left) and between RTA and P2ΔC10 (right).