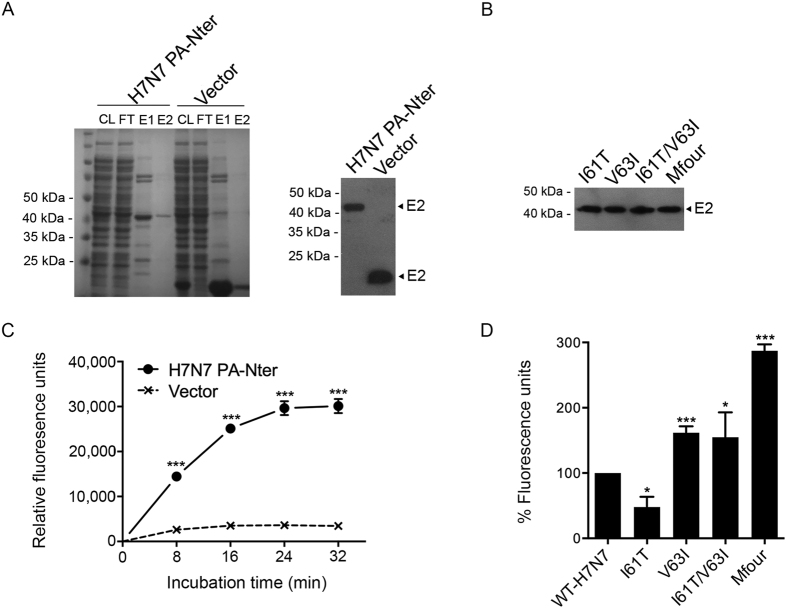
Figure 4. Comparison of the endonuclease activity of virus PA-Nter proteins expressed in E. coli.
Virus PA-Nter proteins were expressed in E. coli, purified, and identified by SDS-PAGE and Western blot using Anti-His antibodies. After concentrated and quantified, the PA-Nter proteins were then incubated with DNA substrates, which contained FAM and BHQ conjugated to the 5′ and 3′ ends, respectively. Fluorescence signals were released and measured at the indicated time points. The purified protein from unmodified vector was achieved and used as a negative control. (A) The Coomassie-stained SDS-PAGE image and Western blot result of WT-H7N7 PA-Nter and negative control protein (Vector). CL, cell lysates; FT, flow-through (wash fractions); E1, 1st eluates; and E2, 2nd eluates. E2 proteins were concentrated and used for the endonuclease assay. (B) Western blot result of purified PA-Nter proteins of the indicated mutants. (C) Endonuclease assay of the WT-H7N7 PA-Nter protein. Data represented are means ± SD of independent experiments performed at least three times. (D) Comparison of the endonuclease activity of the PA-Nter proteins of WT-H7N7 and the indicated mutants. Fluorescence signals were collected at 32 minutes after incubation. The experiments were independently performed at least three times. Results presented are the relative endonuclease activity of PA-Nter proteins of the indicated mutants to that of WT-H7N7 (means ± SD).