Abstract
The relationship between pseudotumor cerebri and contraceptive drugs is controversial. Its association with Implanon, an implantable single-rod contraceptive containing etonogestrel (a progestogen) has not been reported but is the subject of many medico-legal cases. The authors present two case reports of patients using Implanon and who subsequently developed pseudotumor cerebri. Rapid weight gain rather than direct hormonal influence is probably the trigger. Headaches, visual obscurations, and rapid weight gain in patients using Implanon should alert one to the probable diagnosis of pseudotumor cerebri.
Keywords: Implanon, papilloedema, pseudotumor cerebri
INTRODUCTION
Implanon, the implantable contraceptive containing etonogestrel, has been available for use in South Africa since February 2014 in the public and private sectors.1 It is a single rod measuring 40 mm × 2 mm containing 68 mg of etonogestrel and implanted subcutaneously in the medial arm.2 The daily dose released by the implant is sufficient to suppress ovulation for a 3-year period. The implantable route overcomes compliance issues and offers excellent contraception. Replacement is required every 3 years.
Outstanding efficacy has been demonstrated by many studies, with failure usually related to improper implant insertion.3–5 Bhatia et al. reported common adverse events of amenorrhoea, irregular menses, weight gain, acne, and headache.2 Headaches were non-specific and occurred in 7% of users, whereas weight gain of >5 kg was reported in 7.5% over a 3-year period. Menstrual irregularity (polymenorrhagia and amenorrhoea) was the main reason for removal of the Implanon rod and not weight gain.
Much debate exists regarding the role Implanon plays in pseudotumor cerebri (PTC). We report on two patients who acquired PTC soon after having the Implanon rod inserted. Thus far, there is no literature that implicates Implanon directly in the cause of PTC. We propose that it is the rapid weight gain experienced by both patients induced by Implanon, a progestogen-only preparation, that triggers the PTC.
CASES
Patient 1
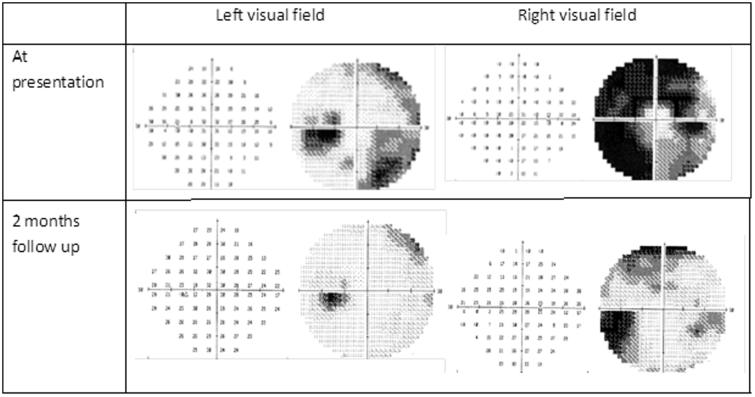
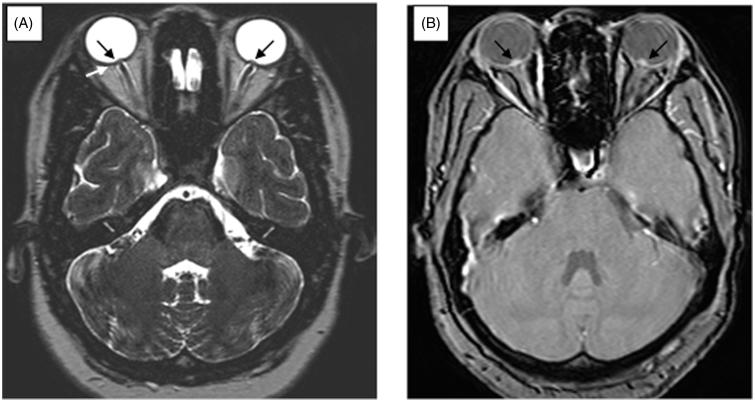
A 24-year-old accounting student presented with a 1-week history of severe holocephalic headache and binocular horizontal diplopia. She awoke each night with severe headaches and photophobia. She had suffered from irregular menses and had Implanon implanted to control them, 4 months before presentation. Following insertion of the device, her weight increased substantially, which she couldn’t quantify but noticed that her dress size increased from size 34 to 36. She was known to be human immunodeficiency virus (HIV) negative and had no other medical history of relevance. On examination, her body mass index (BMI) was 25 kg m−2 (weight of 64 kg) and she was systemically well. She had a convergent squint with limitation of abduction of both eyes and no meningism. Her best-corrected visual acuity was 6/6 OU and pupillary responses were normal. She had bilateral constricted fields and large blind spots on Humphrey’s visual field with the right side more affected (MD −24.28 OD and −10.94 OS) (Figure 1). On funduscopy, she had bilateral and florid papilloedema with exudates and haemorrhages. Her magnetic resonance imaging (MRI) scan of the orbits showed posterior indentation of both optic globes and elevation of the optic nerve head suggestive of papilloedema (Figure 2). The rest of the MRI brain and magnetic resonance venogram (MRV) were normal. Cerebrospinal fluid (CSF) pressure was >50 cm CSF with normal biochemistry and cell count. A diagnosis of PTC was made, and she was commenced on acetazolamide 500 mg twice daily with weight reduction advice offered by the dietitian. The Implanon rod was not removed, as she started to recover almost immediately after the first lumbar puncture. At 3 months follow-up, she was symptom-free, the papilloedema resolved, and VF showed minimal constriction (Figure 1). She maintained her discharge weight despite counselling.
FIGURE 1.
Automated Humphrey’s visual field of patient 1 at presentation and 2 months later, showing significant improvement on the grey scale.
FIGURE 2.
High-resolution T2-weighted (A) and gradient recall echo (B) MRI scan of patient 1 showing posterior indentation of the globes (white arrow) and elevation of the optic nerve head (black arrows) in keeping with papilloedema, bilaterally. No increased tortuosity or dilatation of the optic nerve sheath was noted.
Patient 2
A 21-year-old unemployed, single mother presented with a 6-week history of frontal headaches, photophobia, tinnitus, blurred vision, and transient visual obscurations. She developed bilateral horizontal diplopia prior to admission. Of note, she had the Implanon device implanted 9 months before and gained 20 kg during that period. Her BMI increased from 30 to 38 kg m−2 in those 9 months. She was HIV negative and had no other relevant medical disorders. Her general and systemic examination was normal. Neurological examination revealed normal higher functioning, no neck stiffness, a left sixth nerve palsy, and bilateral severe papilloedema with haemorrhages and exudates. Her best-corrected visual acuity and Humphrey’s visual fields were normal. MRI orbits and brain including MRV were normal. CSF pressure was 26 cm CSF, with normal biochemistry and cell count. Her findings were also consistent with PTC, and she was commenced on acetazolamide 250 mg four times daily, serial lumbar punctures, and weight reduction advice. The Implanon rod was removed 5 weeks later, but recovery was noted to commence even before that. At 2 months follow-up, her tinnitus settled, headaches improved, and papilloedema was present but mild. At 6 months follow-up, she was noted to have made a full recovery despite gaining 2 kg in the interim.
Apart from the recent Implanon implantation and rapid weight gain, neither of these patients had any other exposure to vitamin A preparations, tetracyclines, and non-steroidal anti-inflammatory agents. They had normal serum sodium, potassium, calcium, phosphate, and magnesium levels. Partial thromboplastin time, international normalized ratio, and haemoglobin levels were normal, without evidence of iron deficiency anaemia in both patients. Thyroid function tests and serum cortisol levels were normal. Antinucelar antibodies and rheumatoid factor were negative. Patient 1 had the Implanon inserted to control her irregular menses and patient 2 used it primarily for contraception. Ultrasound of the pelvis in patient 1 showed no polycystic ovarian disease but was not done on patient 2. Neither patient had clinical features of hyperandrogenism, and testosterone levels were not measured.
DISCUSSION
Historically, oestrogen containing oral contraceptives have been associated with PTC, but this association has been tenuous. Early reports suggested a clear connection between the two. Soysa reported on five patients who developed PTC while on oral oestrogen containing contraceptives that reversed after changing to the progesterone-only mini-pill.6 Progesterone appeared to be the safer alternative. Further support for the link between oestrogen and PTC came from case reports only where oestrogen was used for contraception or control of post-menopausal symptoms.7,8 The current opinion regarding oestrogen and PTC is that the early studies might have included undiagnosed patients with cerebral venous sinus thrombosis and that the risk of PTC in women on combined oral contraceptives is similar to that in the general population; a view originally supported in 1991 by Giuseffi et al., who showed no difference in the usage of oestrogen in their 50 patients with PTC to 100 age-matched controls.9,10 However, obesity and recent weight gain were much more common among patients than controls.
Although the evidence for the association between progesterone-only preparations and PTC is absent, that for recent weight gain and PTC is compelling. Ko et al. were able to demonstrate that an average rate of BMI gain of 1.3 kg m−2/year was more likely to result in recurrence than a BMI loss of −0.96 kg m−2/year.11 The rate of weight gain was key. Daniels et al. were able to show that higher levels of weight gain and BMI were associated with greater risk of developing PTC, but in addition, that even non-obese patients (BMI <30 kg m−2) were at greater risk of developing PTC with moderate weight gain,12 once again confirming that the rate of weight gain rather than the final weight is significant.
The onset of PTC in both our patients after having commenced Implanon and who lack all the other associations is striking and suggests a probable association. Patient 1 had normal BMI but gained appreciable weight during the first 4 months, and patient 2 gained 20 kg in 9 months. Rapid weight gain is a common feature of PTC. Whether weight gain or hormonal influences from the Implanon caused the PTC is not verifiable. In patient 1, despite not removing the Implanon rod, she improved promptly with CSF pressure lowering by CSF tapping and acetazolamide, suggesting that the recent weight gain was the trigger. In patient 2, recovery was slower but preceded the removal of the Implanon. Recovery was due to CSF pressure lowering from serial lumbar punctures and acetazolamide rather than the Implanon removal. The common factor in both patients has been the rapid weight gain induced by Implanon.
We propose that the PTC was induced by the rapid weight gain rather than the direct hormonal influence from Implanon in our two cases. Central obesity perhaps increases intra-abdominal pressure, intra-pleural pressure, and central venous pressure, leading to increased intracranial venous pressure.13 However, this mechanism alone does not explain the female predominance in PTC, PTC in thin patients, and the lack of increased incidence in pregnancy. Both our cases support the assertion that it is the speed of weight gain that is pivotal in precipitating PTC rather than the eventual weight of the patient.14 Both patients did not lose weight during follow-up despite the weight losing measures suggested as treatment. Nevertheless, they improved with CSF tapping and acetazolamide. Mechanisms beyond central obesity must play a role and are subjects of ongoing studies.15,16
We do not claim to provide a simple solution to the debate regarding the association between Implanon and PTC by presenting two case reports. Furthermore, sporadic adverse reports can sometimes be rather damaging and this too is not our intention, especially in a developing country where the issue of unwanted pregnancies abounds. It is our intention, however, to point out that both cases do suggest that the tempo of weight gain should be further investigated rather than just obesity in the pathogenesis of PTC. Finally, we would like to recommend that in the setting of Implanon and rapid weight gain, new onset headaches should alert one to the possibility of PTC.
Declaration of interest. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Patel M. Contraception: everyone's responsibility. South Afr Med J 2014;104:8. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia P, Nangia S, Aggarwal S, Tewari C. Implanon: subdermal single rod contraceptive implant. J Obstet Gynaecol India 2011;61:422–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiriwat O, Patanayindee A, Koetsawang S, Korver T, Bennink HJ. A 4-year pilot study on the efficacy and safety of Implanon, a single-rod hormonal contraceptive implant, in healthy women in Thailand. Eur J Contracept Reprod Health Care 1998;3:85–91 [DOI] [PubMed] [Google Scholar]
- 4.Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A long-term study of the efficacy and acceptability of a single-rod hormonal contraceptive implant (Implanon) in healthy women in China. Eur J Contracept Reprod Health Care 1999;4:85–93 [DOI] [PubMed] [Google Scholar]
- 5.Funk S, Miller MM, Mishell DR Jr Archer DF, Poindexter A, Schmidt J, Zampaglione E. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception 2005;71:319–326 [DOI] [PubMed] [Google Scholar]
- 6.Soysa ND. The oral contraceptive pill and benign intracranial hypertension. N Z Med J 1985;98:656. [PubMed] [Google Scholar]
- 7.Sheehan JP. Hormone replacement treatment and benign intracranial hypertension. Br Med J (Clin Res Educ) 1982;284:1675–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finsterer J, Kues EW, Brunner S. Pseudotumour cerebri in a young obese woman on oral contraceptives. Eur J Contracept Reprod Health Care 2006;11:237–240 [DOI] [PubMed] [Google Scholar]
- 9.Ball AK, Clarke CE. Idiopathic intracranial hypertension. Lancet Neurol 2006;5:433–442 [DOI] [PubMed] [Google Scholar]
- 10.Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology 1991;41(2 Pt 1):239–244 [DOI] [PubMed] [Google Scholar]
- 11.Ko MW, Chang SC, Ridha MA, Ney JJ, Ali TF, Friedman DI, Mejico LJ, Volpe NJ, Galetta SL, Balcer LJ, Liu GT. Weight gain and recurrence in idiopathic intracranial hypertension: a case-control study. Neurology 2011;76:1564–1567 [DOI] [PubMed] [Google Scholar]
- 12.Daniels AB, Liu GT, Volpe NJ, Galetta SL, Moster ML, Newman NJ, Biousse V, Lee AG, Wall M, Kardon R, Acierno MD, Corbett JJ, Maguire MG, Balcer LJ. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol 2007;143:635–641 [DOI] [PubMed] [Google Scholar]
- 13.Sugerman HJ, DeMaria EJ, Felton WL III Nakatsuka M, Sismanis A. Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology 1997;49:507–511 [DOI] [PubMed] [Google Scholar]
- 14.Lisk DR, Cummings CC, Charles CC, Foley E, Ujah U. Rapid weight gain and benign intracranial hypertension in an AIDS patient on treatment with highly active anti-retroviral therapy (HAART). West Indian Med J 2000;49:338–339 [PubMed] [Google Scholar]
- 15.Wall M, Kupersmith MJ, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, McDermott M. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol 2014;71:693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batra R, Sinclair A. Idiopathic intracranial hypertension; research progress and emerging themes. J Neurol 2014;261:451–460 [DOI] [PubMed] [Google Scholar]