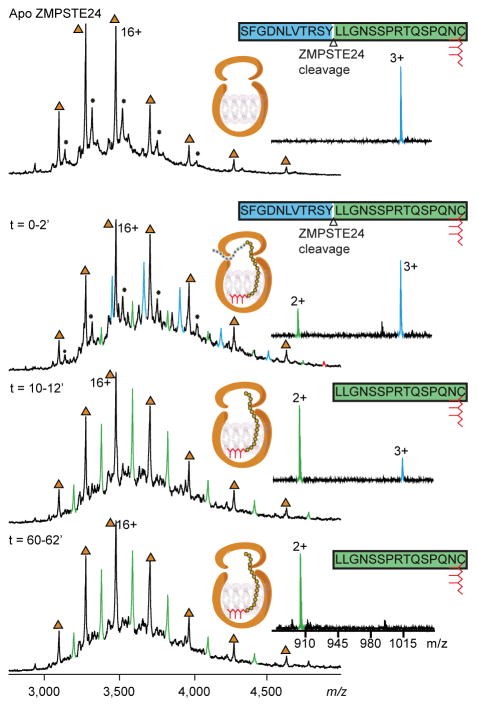
Figure 2.
Mass spectra of ZMPSTE24 allow the cleavage of the prelamin peptide to be monitred in real-time. ZMPSTE24 was preprared in OGNG micelles and incubated with with an equimolar concentration of the peptide the 26-mer prelamin A peptide. Mass spectra were recorded on a Q-Tof mass spectrometer at different time intervals as indicated. Apo protein charge states are labelled (brown triangles). Binding of the full-length 26-mer prelamin A peptide is observed within 2 min (blue peaks). The corresponding 15-mer peptide cleavage product is formed with time (green peaks). The majority of the peptide was cleaved within ~12 min, and the cleaved peptide product remains associated with ZMPSTE24. The schematic for ZMPSTE24 depicts the full-length 26-mer prelamin A peptide and its farnesyl lipid tail (red). Right hand panels show the presence of 26-mer peptide and the cleavage product (1016 for the triply charged ion), indicating that the majority of the substrate peptide was hydrolysed within ~12 min to form the doubly charged product ion at 903 m/z.