Abstract
Human embryonic stem cell line WA01 was genetically modified using zinc-finger nucleases and the PiggyBac/transponson system to introduce a fluorescence reporter for VE-cadherin (VEC; tdTomato) and CD43 (eGFP). Phenotypic and functional assays for pluripotency revealed the modified hES cell reporter lines remained normal. When the cells were differentiated into hematoendothelial lineages, either by directed differentiation or direct reprogramming, flow cytometric and fluorescence microscopy showed that VEC+ endothelial cells express tdTomato and CD43+ hematopoietic progenitors express eGFP.
Endothelial-to-hematopoietic transition (EHT) is a unique process during developmental process that gives rise to blood cells, including hematopoietic stem cells (HSCs). Modeling EHT in a culture in vitro is essential for identifying the molecular program involved in HSC specification. To track EHT from during human embryonic stem cells (hESCs) differentiation into hematoendothelial lineages, we made a VE-cadherin-tTdTomato/CD43-eGFP (VEC-tdTomato/CD43-eGFP) dual reporter fluorescent cell line based on the WA01 hESC line. VE-cadherin (CDH5, CD144) is the one of the most specific marker of endothelial cells [2–4], while CD43 (leukosialin/SPN) is a pan-hematopoietic marker [5] that is expressed by all hematopoietic progenitors during hPSC differentiation cultures [6, 7] and HSCs in the embryo and adult bone marrow [8–10]. A construct containing the CD43 promoter driving eGFP expression [11] was targeted to the AAVS1 locus by ZFN nuclease (Fig. 1A). After integrating the CD43-eGFP construct to the AAVS1 locus, we isolated and selected specific clones that had correct integration of the construct into the AAVS1 locus without random integration as determined by southern blot (Fig. 1B). We subcloned the −3394/+39 VE-cadherin promoter followed by the tdTomato into the PiggyBac vector and co-electroporated the construct with transposons into the selected CD43-eGFP reporter cell clones (Fig. 1A). After isolating of individual VEC-tdTomato/CD43-eGFP dual reporter clones, we selected and expanded clone designated H1.CD43/CD144DR. We confirmed the identity of the generated dual reporter (DR) clonal cell line by Short Tandem Repeat (STR) analysis (Table 1). DR cell line was assayed for pluripotent phenotype by measuring the expression of transcriptional factors, OCT4, SOX2, and NANOG. The DR cell line retained expression of pluripotency markers at the level similar to wild type H1 hESCs (Fig. 1C). As evaluated by embryoid body differentiation, the DR cell line showed capacity to produce all three germ layers following spontaneous differentiation (Fig. 1D). Also, we confirmed that generated DR cell line has normal karyotype (Fig. 1E)
Figure 1.
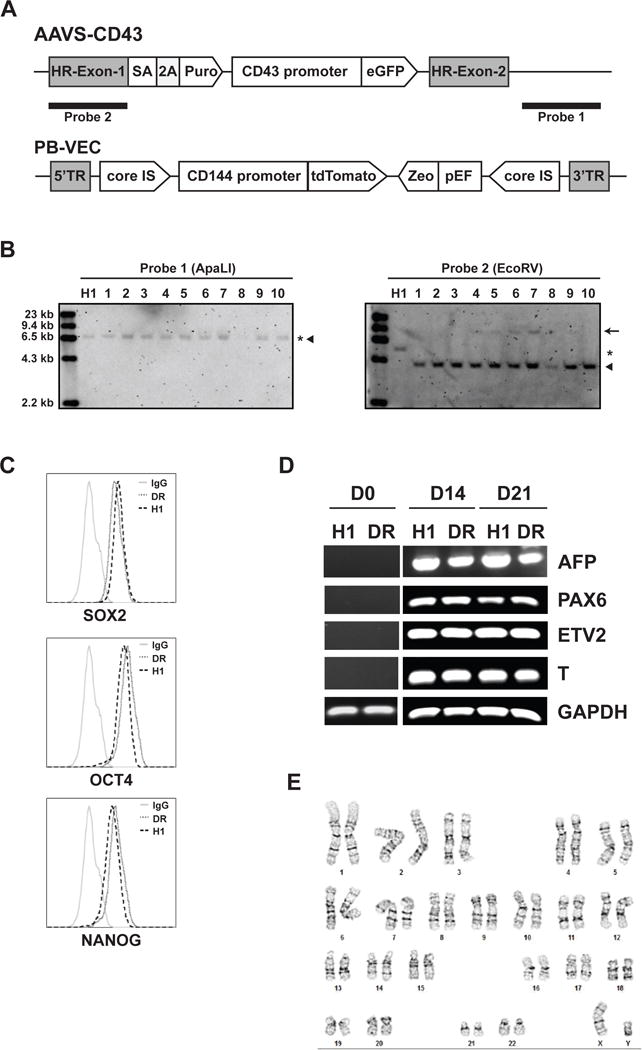
Characterization of H1.CD43/CD144DR dual reporter H1 hESCs. (A) Schematic of the constructs used for targeting of CD43-eGFP reporter into AAVS locus and VEC-tdTomato reporter by PiggyBac system. (B) Southern blot analysis of ApaL1 or EcoRV digested genomic DNA of CD43-eGFP cell. Asterisk, wild type; filled triangle, targeted; arrow, off-targeting. (C) Flow analysis of intracellular staining. Expression of transcriptional factors, OCT4, SOX2, and NANOG, by dual reporter and wild type of hESCs. (D) Differentiation of dual reporter cells to three germ layers. Expression of three germ layers markers AFP (endoderm), PAX6 (ectoderm), ETV2 and T (mesoderm), and GAPDH (internal control) in day 0, day 14 and 21 EBs of dual reporter H1.CD43/CD144DR (DR) cell line and wild type of H1 hESCs analyzed by RT-PCR. (E) Normal Karyotype (46, XY) of generated DR hESC line.
Table 1.
Short Tandem Repeat (STR) profiling of dual reporter with the original cell line.
| STR locus | H1 wild typea | H1.CD43/CD144 Reporter (DR) |
|---|---|---|
| FGA | 20, 24 | |
| TPOX | 8, 11 | 8, 11 |
| D8S1179 | 12, 13 | |
| vWA | 15, 17 | 15, 17 |
| Amelogenin | X, Y | X, Y |
| Penta_D | 10, 13 | |
| CSF1PO | 12, 13 | 12, 13 |
| D16S539 | 9, 13 | 9, 13 |
| D7S820 | 8, 12 | 8, 12 |
| D13S317 | 8, 11 | 8, 11 |
| D5S818 | 9, 11 | 9, 11 |
| Penta_E | 10, 12 | |
| D18S51 | 17, 18 | |
| D21S11 | 28, 32.2 | |
| TH01 | 9.3, 9.3 | 9.3, 9.3 |
| D3S1358 | 15, 15 |
STR data obtained from Wicell (http://hpscreg.eu/docs/uploads_link/certificate_of_analysis/28f8d7dc69240e58dad1083d492983bf.pdf).
To determine the specificity and functionality of the DR cell line, we differentiated it for 8 days in chemically defined conditions [12] and analyzed for the expression of VEC and CD43 with antibodies and their respective fluorescent protein. Typically, VEC+ hemogenic endothelial progenitors are first detected on day 4 of differentiation, while CD43+ hematopoietic progenitors can be detected from day 5 of differentiation (Fig. 2A). Fluorescence microscopy showed tdTomato and eGFP were detected from day 4 and day 5, respectively, and continued through day 8 (Fig. 2A, B). Flow cytometry analysis revealed the tdTomato+ cells were exclusively detected in the VEC+ endothelial population (Fig. 2B) while GFP+ cells were almost exclusively detected in CD43+ hematopoietic cells (Fig. 2B). Consistent with the current understanding of hematopoietic development, CD43-GFP+ cells arose from VEC-tdTomato+ hemogenic endothelial cells from day 5.
Figure 2.
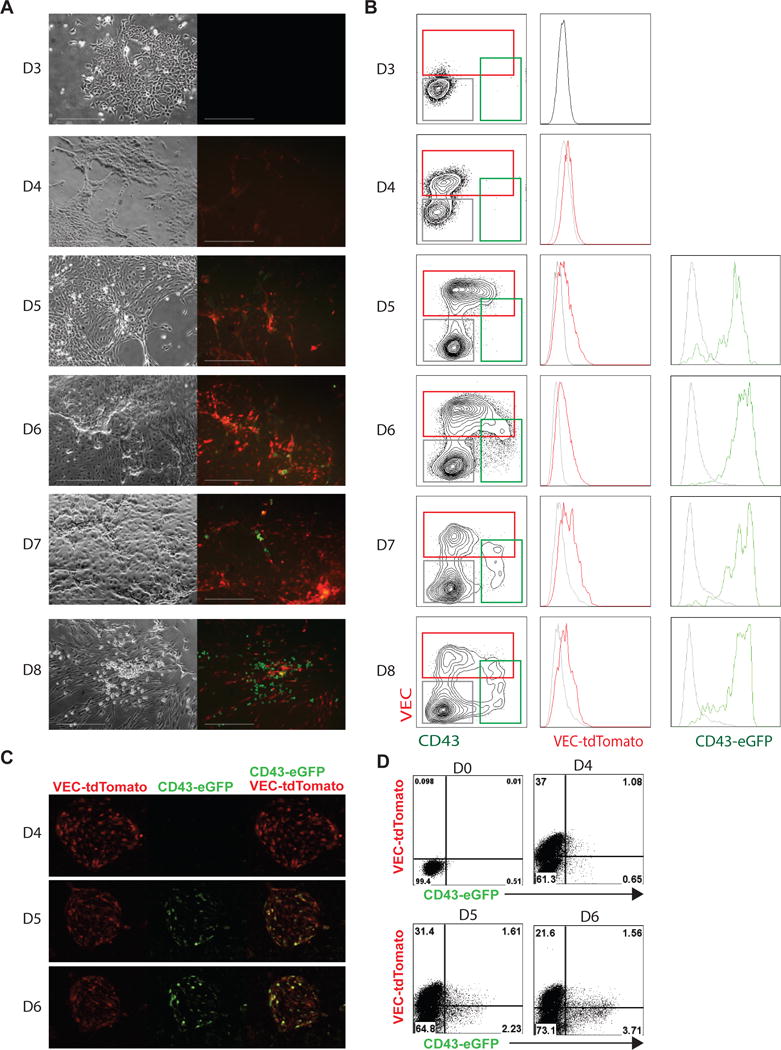
Differentiation of dual reporter cells to three germ layers and hematopoietic cells. Kinetic analysis of CD43-eGFP and VEC-tdTomato expressed during hematopoiesis by fluorescence microscopy (A) and flow cytometry (B). Cells were differentiated and checked for eGFP and tdTomato fluorescence everyday from day 3 to day 8. VEC-reporter cell expressed tdTomato and CD43-reporter cell expressed eGFP. Scale bar is 300 μM. Gray histogram is gated on the CD43−VEC− population, green histogram is gated on the CD43+ population and represents eGFP expression, and red histogram is gated on the VEC+ population and represents tdTomato expression. (C) GATA2+ETV2 induced cells with endothelial morphology can give rise to blood cells. Transition of VEC expressing cuboidal cells (endothelial cells) into round hematopoietic cells is associated with the loss of VEC expression (red) and acquisition of CD43 expression (green). (D) Cells were treated with GATA2+ETV2 virus and checked for CD43-eGFP and VEC-tdTomato at day 0, 4, 5, and 6 by flow cytometry.
Recently we demonstrated that overexpression of ETV2 and GATA2 in undifferentiated hPSCs induces the formation of hemogenic endothelium which undergoes EHT [13]. Following infection of VEC-tdTomato/CD43-GFP dual reporter hESCs with ETV2 and GATA2-expressing lentiviruses, we observed expression of tdTomato+ within the endothelial population which subsequently underwent transition to eGFP+ blood cells between days 4 and 6 (Fig. 2C and 2D and supplementary movie 1). Overall, these results demonstrate that the dual VEC-tdTomato/CD43-eGFP reporter hESC line allows for easy visualization of EHT and provides a useful tool for assessing molecular factors involved in EHT regulation.
Materials and Methods
Maintenance and Hematopoietic differentiation of hESCs
H1 (WA01, Wicell, Madison, WI) hESCs were cultured on vitronectin (Stem Cell Technologies), in E8 medium [14]. Cells were passaged every 5 days (80% confluency) using 0.5 mM EDTA in PBS. hESCs were differentiated in collagen IV-coated plate as previously described [12].
Vector construction
ZFN vectors targeting the AAVS1 locus and AAVS1-SA-2A-PURO-CD43 promoter-eGFP vector are kindly provided by Paul Gadue [11]. The VE-Cadherin promoter-tdTomato construct was cloned to Piggybac transposon vector (Transposagen). The VE-Cadherin −3394/+39 promoter region was amplified by PCR from BAC clone.
Gene targeting of the AAVS1 locus and PiggyBac system
Cells were dissociated into single cells by treatment with TrypLE (Life technologies). One million cells were resuspended in 100 μl reagent (1 × 106 cells) of Amaxa human stem cell nucleofector kit 2 (Lonza) with 1 μg ZFN-left and right plasmid each and 10 μg AAVS targeting plasmid. For targeting PiggyBac, 0.5 μg transposase plasmid and 5 μg transposon plasmid were electroporated using program A-13 according to manufacture protocol (Amaxa). The electroporated cells were resuspended with E8 (Stem Cell Technologies) culture medium and rock inhibitor (10 μM, Tocris Y-27632) and then they were plated and cultured with E8 growth medium in 6-well plate. Puromycin and Zeocin selection (0.5 μg/ml, Life technologies) was started 3 days after electroporation. After 10 days, surviving colonies were singularized and sorted to 96 well plate as single cells, from which they were expanded individually.
Southern blot
Southern blot (SB) analysis was performed, as described in the protocol included in the DIG-labeling hybridization (Roche). Briefly, 10 μg genomic DNA was digested using restriction enzymes for O/N, separated on a 0.7% agarose gel, transferred to a nylon membrane (Amersham) with DIG-labeling probes. The external probe 1 was 600 base pair ApaLI fragment in 5′ external region, and internal probe1 was 600 base pair EcoRV fragment in internal region of AAVS-CD43 promoter-GFP.
Embryonic body (EB) formation
Cell harvested and cultured in ultra-low attachment well (Corning) to induce EB formation. EBs were cultured for 21 days with E6 media. Medium was changed every 2–3 days. EB were harvested at 14 days and 21 days and extracted RNA and analysis by RT-PCR with marker of three germ layers (Table 2).
Table 2.
Primer sequences for three germ layers
| Target | Forward primer | Reverse primer |
|---|---|---|
| PAX6 | GAAGATGGTGATGGGATTTC | CGTTGGACACGTTTTGATTG |
| AFP | GAATGCTGCAAACTGACCACGCTGGAAC | TGGCATTCAAGAGGGTTTTCAGTCTGGA |
| ETV2 | TCTTTGAAGCGGTACCAGAG | GGGACCTCGGTGGTTAGTT |
| T | GACAATTGGTCCAGCCTTG | GGGTACTGACTGGAGCTGGT |
| GAPDH | GAAGGTGAAGGTCGGAGT | GAAGATGGTGATGGGATTTC |
Flow cytometry
Cells were dissociated into single cells by treatment with 1× Tryple. Cells analyzed using MACSQuant 10 (Miltenyi Biotech). Antibodies used included CD43-APC-Vio770 (Miltenyi Biotech) and VEC-VioBlue (Miltenyi Biotech). Intracellular staining to determine OCT4 (BD), NANOG (BD), and SOX2 (BD) expression was performed using FIX & PERM cell permeabilization reagents (BD).
Time-Lapse Microscopy
Endothelial to hematopoietic transition (EHT) was monitored by time-lapse microscopy using fluorescent optics. H1ESCs line expressing VE-Cadherin-Td tomato and CD43-GFP dual reporter were transduced with GATA2+ETV2 lentiviral constructs and cultured for 4 days until endothelial cluster were formed [13]. Time-lapse confocal imaging was performed over two days to capture blood formation. Time lapse movies were recorded using Nikon Eclipse Ti-E configured with an A1R confocal system, motorized stage (Nikon Instruments Inc. Melville, NY), and Tokai-Hit Stage Top Incubator (Tokai Hit CO., Ltd., Shizuoka-ken, Japan) at 37 °C and 5% CO2. Images were acquired using Nikon Elements (NIS – element C) imaging software for every 5 min with CFI Plan Fluor DLL 10× NA 0.5 WD 2.1MM objective (Nikon Instruments Inc. Melville, NY). The time-lapse serial images were converted to Quick-time movies (.mov) and analyzed using ImageJ software (NIMH, Bethesda, MD).
Supplementary Material
Acknowledgments
We thank Dr. Gadue (The Children’s Hospital of Philadelphia) for providing AAVS1-SA-2A-PURO-CD43 promoter-eGFP vector. This work was supported by funds from the National Institute of Health (R01HL116221, U01HL099773, and P51 OD011106) and The Charlotte Geyer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomson J, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel J, Marshall V, Jones JM. Embryonic Stem Cell Lines Derived from Human Blastocysts.pdf. 1998 doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87:630–641. [PubMed] [Google Scholar]
- 3.Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remold-O’Donnell E, Zimmerman C, Kenney D, Rosen FS. Expression on blood cells of sialophorin, the surface glycoprotein that is defective in Wiskott-Aldrich syndrome. Blood. 1987;70:104–109. [PubMed] [Google Scholar]
- 6.Choi KD, Vodyanik M, Slukvin II. Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat Protoc. 2011;6:296–313. doi: 10.1038/nprot.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore T, Huang S, Terstappen LW, Bennett M, Kumar V. Expression of CD43 on murine and human pluripotent hematopoietic stem cells. J Immunol. 1994;153:4978–4987. [PubMed] [Google Scholar]
- 9.Rybtsov S, Batsivari A, Bilotkach K, Paruzina D, Senserrich J, Nerushev O, Medvinsky A. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(−) embryonic precursor. Stem cell reports. 2014;3:489–501. doi: 10.1016/j.stemcr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inlay MA, Serwold T, Mosley A, Fathman JW, Dimov IK, Seita J, Weissman IL. Identification of multipotent progenitors that emerge prior to hematopoietic stem cells in embryonic development. Stem cell reports. 2014;2:457–472. doi: 10.1016/j.stemcr.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiyaboonchai A, Mac H, Shamsedeen R, Mills JA, Kishore S, French DL, Gadue P. Utilization of the AAVS1 safe harbor locus for hematopoietic specific transgene expression and gene knockdown in human ES cells. Stem Cell Res. 2014;12:630–637. doi: 10.1016/j.scr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uenishi G, Theisen D, Lee JH, Kumar A, Raymond M, Vodyanik M, Swanson S, Stewart R, Thomson J, Slukvin I. Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem cell reports. 2014;3:1073–1084. doi: 10.1016/j.stemcr.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elcheva I, Brok-Volchanskaya V, Kumar A, Liu P, Lee JH, Tong L, Vodyanik M, Swanson S, Stewart R, Kyba M, Yakubov E, Cooke J, Thomson JA, Slukvin I. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat Commun. 2014;5:4372. doi: 10.1038/ncomms5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


