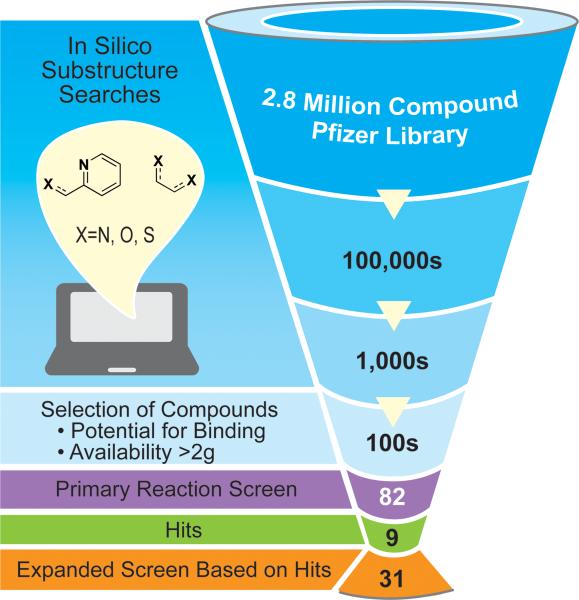
Fig. 2.
Strategy employed for mining the Pfizer compound library for new ligands. Substructure searching of the 2.8 million compound library resulted in hundreds of thousands of hits. Refining these hits to motifs that looked likely to bind and were available in sufficient quantity narrowed this number to hundreds. Of this number, we screened 82 compounds against a typical cross-electrophile coupling reaction (Fig. 1a), resulting in nine hits with comparable yield and selectivity to dmbpy (C1). Based upon the nine hits, the compound library was mined again to generate a secondary library of 31 ligands used in further testing against more challenging reactions (Fig. 3).