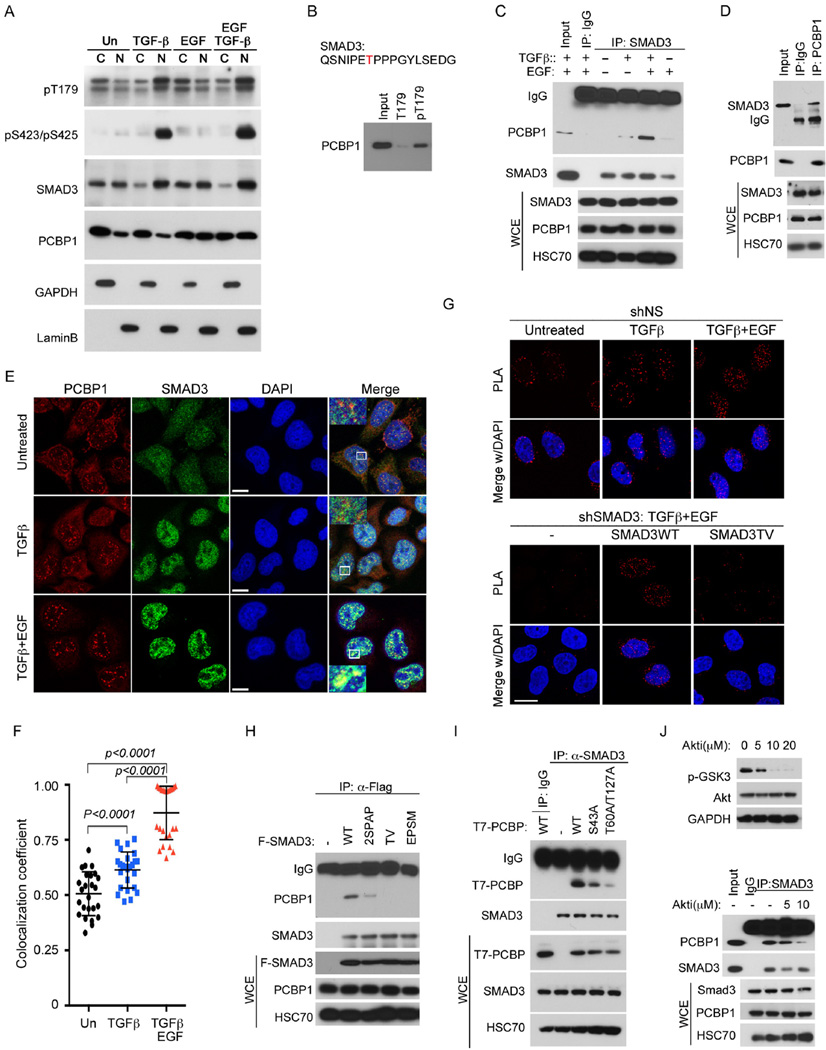
Figure 2. Phosphorylation-dependent interaction between SMAD3 and PCBP1.
A: Nuclear and cytosolic distribution of SMAD3 and PCBP1 in HeLa cells treated with indicated growth factors for 2 hr. GAPDH and Lamin B were used as cytoplasmic (C) and nuclear (N) specific protein markers, respectively.
B: Western blot of PCBP1 isolated from HeLa cells nuclear extracts using immobilized SMAD3 T179 or pT179 peptides.
C: Western blots of SMAD3 and PCBP1 following immunoprecipitation (IP) of SMAD3 from whole cell extracts (WCE) of HeLa cells treated with TGF-β and/or EGF for 2 hr.
D: Western blots of SMAD3 and PCBP1 following IP of PCBP1 from WCE of HeLa cells treated with TGF-β and EGF for 2 hr.
E: Immunofluorescence staining of SMAD3 and PCBP1 in HeLa cells treated with TGF-β or TGF-β and EGF for 2 hr. The yellow color is indicative of colocalization. Bar =10 µm.
F: Scatter dot plot of co-localization coefficiency of SMAD3 with PCBP1 from (E). The data are shown as mean ± SD (n=25).
G Proximity ligation assay (PLA) of SMAD3 and PCBP1 in the nucleus of HeLa cells. TGF-β or combined TGF-β and EGF treatments were for 2 hr. Bar = 20 µm.
H: IP-Western analyses of various Flag-tagged SMAD3 mutants to bind endogenous PCBP1 in HeLa cells after treatment with TGF-β and EGF for 2 hr.
I: IP-Western analyses of various T7-tagged PCBP1 mutants to bind endogenous SMAD3 in HeLa cells after treatment with TGF-β and EGF for 2 hr.
J: AKT inhibitor at a concentration (10 µM) that is sufficient to block GSK3β phosphorylation (top) decreased the affinity of SMAD3 to PCBP1 in HeLa cells treated with TGF-β and EGF for 2 hr (bottom).
See also Figure S2.