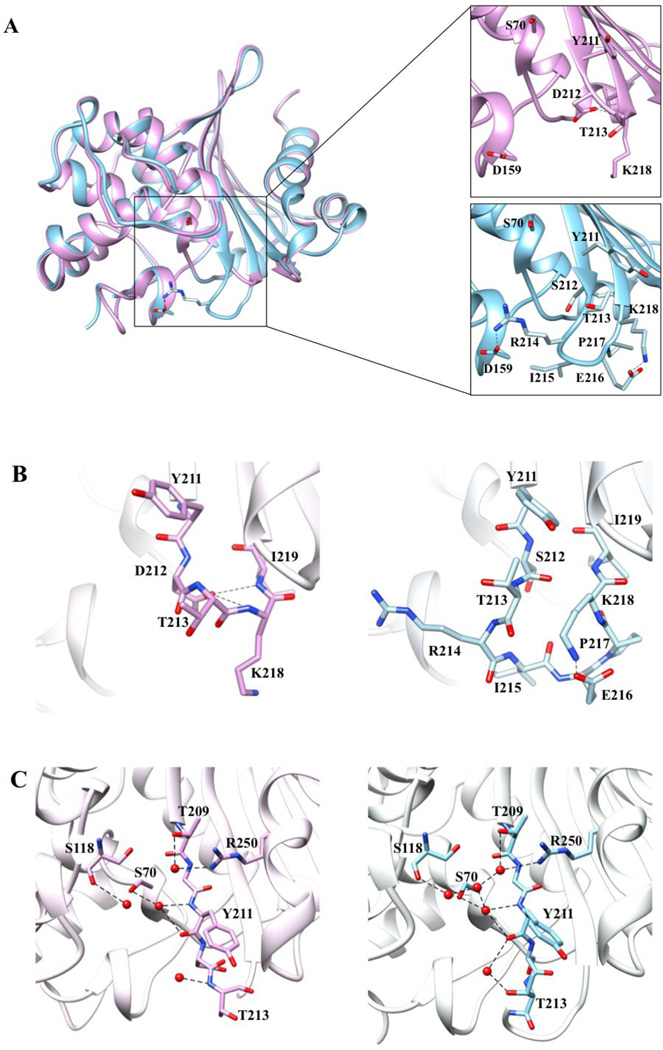
Figure 2. Comparison of the tertiary structures of OXA-48 at 1.9 Å and OXA-163 at 1.72-Å resolution.
A) Cα alignment of OXA-48 (blue) and OXA-163 (lavender). The insets provide a detailed view of the β5–β6 loop region residues represented in stick model with black dashes representing interactions. Arg214 in OXA-48 forms an electrostatic interaction with Asp159 located on the Ω-loop. This interaction confines the bottom boundary of the active-site cavity in OXA-48. In OXA-163 Arg214 is absent and so the interaction is lost, resulting in an expanded active-site cavity. B) Stick representation of the β5–β6 loop region of OXA-48 and OXA-163. The OXA-163 residues that undergo the largest movement compared to OXA-48 are Tyr211, Thr213, and Lys218. The S212D substitution results in newly formed hydrogen bonds between the Asp212 side chain and the -NH main chain of Lys218 and Ile219 represented as black dashed lines. C) Active-site hydrogen bond network in OXA-48 and OXA-163. Several water molecules exhibit altered positions or are missing in OXA-163 compared to OXA-48. Distances of 2.5–3.8 Å are shown as dashed black lines.