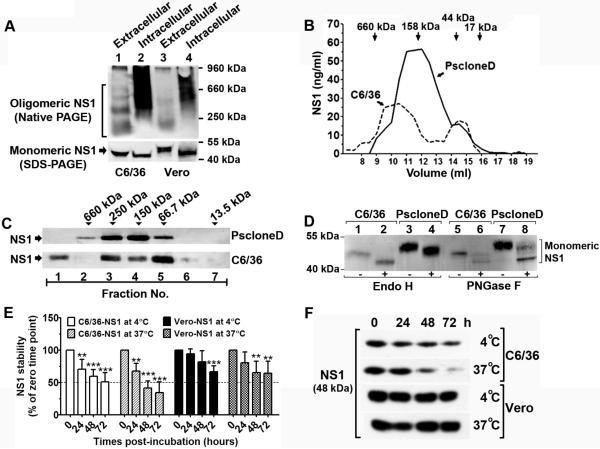
FIGURE 3. Oligomeric assembly, glycosylation pattern and stability of NS1 secreted from DENV-infected insect cells.
Fifty-fold concentrated supernatants (lanes 1 and 3) and cell lysates (lanes 2 and 4) from DENV-infected C6/36 cells (lanes 1 and 2) and Vero cells (lanes 3 and 4) were separated by Native-PAGE (A, upper panel) and reducing 10% SDS-PAGE (A, lower panel) and Western blotted with 1F11 DENV NS1 specific mAb. B. Fifty-fold concentrated DENV-2 infected C6/36 (dashed line) and PscloneD (solid line) supernatants were passed over a Superdex 200 column and fractions were collected and quantitated for NS1 concentration by NS1-ELISA. Molecular weights of reference protein standards fractionated in parallel are depicted. C. Fifty-fold concentrated DENV-infected C6/36 or PscloneD supernatant was loaded on a 5% to 55% sucrose gradient. NS1 in each fraction was detected by Western blotting with 1F11 anti-NS1 mAb. Arrowheads indicate molecular weights of protein standards. D. Endoglycosidase (Endo H or PNGase F) treatment of extracellular NS1 derived from C6/36 (lanes 1, 2, 5 and 6) and PscloneD (lanes 3, 4, 7 and 8) cells prior to Western blot analysis with 1F11 anti-NS1 mAb. E-F. NS1 stability test.Supernatants from DENV-infected C6/36 and Vero cells were incubated in the presence of a protease inhibitor (10 mM PMSF) at 4°C or 37°C for 3 days. Amounts of NS1 at each time point of C6/36 cell-derived or Vero cell-derived at 4°C and 37°C were determined by ELISA and calculated by normalizing the zero-time point of each condition to 100 percent (E) and by Western blotting with 1F11 anti-NS1 mAb (F). Error bars indicate SD corresponding to three independent experiments. Values that are significantly different from the value for zero-time point are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.