Abstract
Individuals with chronic kidney disease (CKD), especially older adults, are at more risk of experiencing cognitive impairment, possibly leading to mild cognitive impairment (MCI) and/or dementia. Studies report associations between CKD and cognitive impairment; although unclear, there seems to be a graded association between stage of CKD and affected cognitive domains, with executive function being affected earlier in the process than episodic memory and global ability. In CKD, dysxecutive MCI and vascular dementia are also more prominent than other subtypes. Explanations are directed towards traditional and non-traditional vascular factors, which may also explain or mediate the association between CKD and type of cognitive impairment. Future research is urged to focus on the longitudinal association between specific domains of cognitive function, including executive function and memory and CKD; to develop screening tools fit for every CKD stage in elderly individuals, and lastly, to use imaging methods that may help clarify the underlying mechanisms connecting the kidney and the brain.
Keywords: chronic kidney disease, albuminuria, cognitive impairment, MCI, dementia
1. Introduction
The prevalence of CKD increases with age, with an estimate of 47% of individuals of 70 years and older in the United States affected by the disease (1). Kidney and cognitive function are associated, to the extent that in the case of chronic kidney disease (CKD) and albuminuria, mild cognitive impairment (MCI) is often prevalent (2-9). In this review we examine studies by domain of cognitive function and CKD stage and its relationship to cognitive states, including MCI and dementia; we also discuss current theories on this association, and suggestions for future research, which include clarifying the specific associations between these two processes and their etiologies to help in developing effective ways of reducing cognitive decline.
CKD is typically defined by decreased levels of estimated glomerular filtration rates (eGFR < 60ml/min/1.73m2) or by increased levels of albuminuria (≥30mg of albumin per 1g of creatinine) for 3 months or more (10). CKD is further subdivided into five stages that are linked to graded stages of severity. Stages one (eGFR ≥90 ml/min/1.72m2) and two (eGFR 60 – 89 ml/min/1/.72m2) indicate normal and slightly decreased kidney function in the presence of albuminuria. Stage three (eGFR 30 – 59 ml/min/1/.72m2) indicates moderate CKD and can be divided into Stage 3a (eGFR 45 – 60ml/min/1.73m2) and stage 3b (eGFR 30 – 45 ml/min/1.73m2). Stages four (eGFR 15 – 29 ml/min/1/.72m2) and five (eGFR <15 ml/min/1.72m2) indicate severe kidney damage and end-stage renal disease (ESRD), respectively. In a meta-analysis covering over 2 million individuals between the ages of 18 and 108 from Asia, Australia, Europe and North and South America, low eGFR and high albuminuria independently predicted mortality risk and incident ESRD (11). Low and declining eGFR is also associated with cognitive impairment (3, 4, 6, 8, 9, 12), cognitive decline (13), and incident MCI and dementia (2, 14).
2. Search strategy
The aim of our review was to summarize some of the existing literature on the association between kidney function and cognition in old age. We performed a search of PubMed for our searches using the keywords “kidney function”; “kidney”; “cognitive function”; “albuminuria”; “cognition”; and “older adults”. Although this article was not a systematic review, we selected literature based on the following criteria:
- Included participants at 60 years and older.
- Included measures of both kidney function and cognitive function.
- Reported in English.
Articles were reviewed for relevance by abstract. The final list of references was based on the scientific quality and relevance to our topic for review. We excluded studies that involved trials or interventions.
3. Cognitive impairment in CKD
Cognitive function is affected by normative aging, by chronic illness, and by morbidity, among other factors. In CKD, cognitive impairment appears early on in the course of the disease (3, 6). Research shows that in mild CKD, executive function and attention are most affected, while in severe CKD (e.g. Stages 4 and 5), cognitive impairment is more global and more severe (7, 13), possibly because individuals are even older by the time they reach this stage with widespread effects of the disease. However, all three major domains of cognition (general cognitive abilities, sometimes also referred to as global, executive function, and episodic memory) are affected in CKD, albeit in different stages. In Table 1 we summarize results of a list of studies included in this review and which results we refer to in the follow sections.
Table 1.
Summary of studies examining the association between chronic kidney disease and cognitive outcomes included in this review.
| Author/date | Study sample | Study design: N; age | CKD/Albuminuria cutoffs | Cognitive outcomes | Main result |
|---|---|---|---|---|---|
| Barzilay, 2008 | Cardiovascular Health Cognition Study | Cross-sectional: 2,316; ≥65 | Albuminuria: Cr ≥ 30 mg/g | MCI; dementia | Risk of dementia increased in the presence of albuminuria |
| Barzilay, 2011 | ONTARGET/TRANSCEND Studies | Longitudinal: 28,384; ≥65 | Microalbuminuria: Cr: 30 - 299 mg/g; Marcoalbuminuria: Cr ≥300mg/g | Cognitive decline | Participants with albuminuria were more likely to have a poorer MMSE score than those without albuminuria. On follow-up, participants with baseline albuminuria had higher risk of cognitive decline compared with those with no albuminuria. |
| Bruce, 2008 | Fremantle Diabetes Study | Longitudinal: 205; ≥70 | ARC ≥3.0mg/mmol | Cognitive decline | Albuminuria predicted cognitive decline independent of covariates. |
| Buchman, 2009 | Rush Memory and Aging Project | Longitudinal: 886; >70 | CKD: eGFR<60mL/min/1.73m2 | Cognitive function | Declines in renal function were associated with declines in sematic memory, episodic memory, and working memory, but not with perceptual speed or visuospatial abilities. |
| Elias, 2009 | Main-Syracuse Longitudinal Study | Cross-sectional: 923; >40 | CKD: eGFR<60mL/min/1.73m2 | Cognitive function | Participants with CKD who were also in the lowest quartile of cognitive scores group had a higher risk of impairment in domains of visual-spatial organization, scanning and tracking, and on global cognition than participants with no CKD, independent of CVD risk factor. |
| Etgen, 2009 | The Intervention Project on Cerebrovascular Disease and Dementia | Longitudinal: 396; ≥55 | CKD stages: eGFR <45, 45-59, ≥60 mL/min/1.73m2 | Incident MCI | Subjects with moderate-to-severe kidney disease (eGFR 45 – 59ml/min/m2) at baseline were more likely to develop new cognitive impairment after the 2-year follow- up. |
| Helmer, 2001 | The Three-City Study | Longitudinal: 7,839; ≥65 | CKD: eGFR<60mL/min/1.73m2 | Incident dementia | No increased risks of cognitive decline or dementia were associated with low eGFR level. More accelerated eGFR decline was associated with global cognitive decline and incident vascular dementia. |
| Jassal, 2010 | Rancho Bernardo Study. | Longitudinal: 759; ≥50 | Albuminuria: ACR of ≥30 mg/g | Cognitive function | Baseline albuminuria in males, but not in females, was associated with reduced performance on all tests of cognitive function at follow-up. An ACR of ≥30 mg/g was associated with greater annual decline in the MMSE and category fluency scores. There were no associations for eGFR. |
| Joosten, 2011 | Prevention of Renal and Vascular End-Stage Disease | Cross-sectional: 4095; 34 – 82 | Albuminuria: <10, 10 to 29, and ≥30 mg/24 h | Cognitive function | Albuminuria and poor cognitive function was associated in young but not in old participants. There was no association between eGFR and cognitive function. |
| Kuo, 2007 | National Health and Nutrition Examination Survey 1999-2002 | Cross-sectional: 2049; ≥60 | Microalbuminuria: 30 – 300 mg(−1) | Cognitive function | Presence of microalbuminuria was inversely associated with cognitive function. |
| Kurella Tamura, 2011 | Reasons for Geographic and Racial Disparities in Stroke (REGARDS) Study | Longitudinal: 19,399; ≥45 | Urine ARC <10, 10-30, 30-300, ≥300mg/g. eGFR<60mL/min/1.73m2 | Incident cognitive impairment | In participants with ACR <10mg/g and with eGFR <60 ml/min/1.73m2 the risk for incident cognitive impairment was significantly higher when compared to those with eGFR ≥60 ml/min/1.73m2 and ACR ≥10mg/g. |
| Kurella, 2005 | The Health, Aging and Body Composition Study | Longitudinal: 3,075; 68 – 80 | eGFR <45, 45-59, ≥60 mL/min/1.73m2 | Cognitive impairment | More advanced stages of CKD (eGFR<45) were associated with increased risk for cognitive impairment and decline independent of confounders. |
| Kurella, 2005 | The Heart Estrogen/Progestin Replacement Study | Cross-sectional: 1,015; <80 | eGFR <45, 45-59, ≥60 mL/min/1.73m2 | Cognitive function | The odds of scoring poorly on Trails B, Boston Naming Test, and Word List Memory immediate recall increased with lower eGFR. |
| Kurella, 2004 | Clinical sample | Cross-sectional: 160; ≥60 | eGFR<60mL/min/1.73m2 | Cognitive function | A graded association was present between cognitive function and severity of CKD. Cognition function was significantly worse in ESRD than in CKD. |
| Madan, 2007 | Clinical Sample | Cross-sectional: 55; 31 – 32 | eGFR <15, 16-45, 45-59, ≥60 mL/min/1.73m2 | Processing speed:P3 event-related potentials. | Speed of processing (P300 latency) was significantly slower with severity of CKD from stage 3, to stage 4, to stage 5. |
| Post, 2010 | Clinical sample | Cross-sectional: 51; 39 – 87 | Stage III CKD: eGFR 30–59 ml/min/1.73m2, stage IV CKD was defined as eGFR 15–29 ml/min/1.73 m2 | MCI | Most common cognitive deficits were non-amnestic in nature and involving multiple domains (attention and processing speed, executive function, and language). |
| Sajjad, 2012 | Nurses’ Health Study | Longitudinal: 1,700; ≥70 | GFR <60 ml/min per 1.73 m2 or an ACR ≥5 mg/g. | Cognitive decline | A higher ARC was associated with 2-7 times more rapid decline in cognitive domains of general cognition, verbal memory, verbal fluency, and working memory; no associations were found between eGFR and cognitive decline. |
| Sànchez-Romàn, 2010 | Clinical sample | Cross-sectional: 161; 17 – 65 | eGFR; Stage 1: >90; stage 2: 56–89; stage 3:30–59; stage 4:15–29; stage 5: <15mL/min per 1.73m2 | Cognitive function | A graded association was found between CKD stage and cognitive performance, with patients with more severe CKD stages performing worse. |
| Seliger, 2004 | Cardiovascular Health Cognition Study | Longitudinal: 3,349; ≥65 | Inverse of serum creatinine 1/SCr. Elevated SCr: ≥1.3 mg/dl if female or ≥1.5 mg/dl if male | Incident dementia | Elevated serum creatinine was associated with a higher risk of incident dementia among participants with good baseline health. |
| Seliger, 2015 | Baltimore Longitudinal Study of Aging | Longitudinal: 2,116; ≥60 | Inverse of serum creatinine 1/SCr. Elevated SCr: ≥1.3 mg/dl if female or ≥1.5 mg/dl if male and eGFR <60 ml/min per 1.73 m2 | Cognitive decline | Declining renal function was associated with a more rapid decline in verbal learning and memory, and visual memory. |
| Slinin, 2008 | Osteoporotic Fractures in Men Study | Longitudinal: 5,529; ≥65 | eGFR <45, 45-59, ≥60 mL/min/1.73m2 | Cognitive impairment and decline | An association was reported between poor executive function and renal impairment at baseline but not with global cognitive impairment. A longitudinal association between cognitive and kidney function was absent. |
| Szerlip, 2015 | Health and Aging Brain among Latino Elders | Cross-sectional: 437; 50 – 91 | eGFR <45, 45-59, ≥60 mL/min/1.73m2 | Cognitive performance | Participants with an eGFR <45 ml/min/1.73m2 performed significantly worse on tasks of processing speed, executive function, visuospatial skills, and global cognitive function than participants with eGFRs of 45-59 and ≥60 ml/min/1.73m2. They also performed significantly worse than those with eGFR ≥60 ml/min/1.73m2 on delayed memory. |
| Thorton, 2007 | Clinical sample | Cross-sectional: 106; 38 – 89 | eGFR ≥60 mL/min/1.73m2 | Verbal memory; executive function | Subjects with CKD had poorer scores on measures of verbal memory and executive functioning in comparison with healthy controls. |
| Vupputuri, 2008 | National Health and Nutrition Examination Survey 1999–2002 | Cross-sectional: 2,386; ≥60 | Microalbuminuria: ACR: 17–250 mg g−1 in men and 25–350 mg g−1 in women | Digit Symbol Substitution Test | Albuminuria and poor cognitive function were more strongly correlated in participants with peripheral artery disease. |
| Weiner, 2009 | 4 Boston-area homecare agencies | Cross-sectional: 335, ≥60 | Microalbuminuria: ACR: 17-250 mg/g in Males and 25-350mg/g in Females | Executive function; memory | Albuminuria was associated with worse executive function but not with worse memory or working memory. Individuals with albuminuria were more likely to be in the lower versus the highest tertile of executive functioning. |
| Yaffe, 2010 | Chronic Renal Insufficiency Cohort | Cross-sectional: 825; ≥55 | eGFR <30, 30-44, 45-59, ≥60 mL/min/1.73m2 | Cognitive performance | Participants with advanced CKD (eGFR<30 ml/min/1.73m2) were more likely to have significant impairment on global cognition, naming, attention, executive function, and delayed memory, but not on category fluency than those with mild to moderate CKD (eGFR 45–59). |
| Zammit, 2015 | Einstein Aging Study | Cross-sectional: 649; ≥70 | eGFR: low <45, moderate: 45-59, high ≥60 mL/min/1.73m2 | Cognitive performance | Poor frontal executive function was significantly associated with the low eGFR; episodic memory and general ability were not associated with any eGFR groups. |
| Zammit, 2015 | Einstein Aging Study | Cross-sectional: 622; ≥70 | eGFR <45, 45-59, ≥60 mL/min/1.73m2 | aMCI; dysexecutive function. | Poor eGFR (<45) was associated with both aMCI and dysexectuvie function. |
Note: CR = Creatinine; CKD = Chronic Kidney Disease; ESRD = end stage renal disease; ACR = Albumin Creatinine Ratio; eGFR = estimated glomerular filtration rate; MCI = mild cognitive impairment; aMCI = amnestic mild cognitive impairment; MMSE= Mini Mental State Examination.
3.1. General/Global cognitive impairment
At cross-section, CKD has been associated with a higher risk of cognitive impairment at baseline using the Modified Mini Mental State Examination (3MS) in 3,075 elderly individuals in the Health, Aging and Body Composition Study (3). In this study, individuals with an eGFR between 45 and 59 ml/min/1.73m2 were just as likely to experience cognitive impairment as individuals with an eGFR lower than 45ml/min/1.73m2. This association was significant after adjusting for age, race, level of education, cardiovascular disease risk factors, hypertension and diabetes. In the Chronic Renal Insufficiency Cohort (CRIC), 825 participants over the age of 55 and with advanced CKD (eGFR 30-44 ml/min/73m2) and ESRD (eGFR <30ml/min/73m2) had higher odds of cognitive impairment measured by the 3MS (OR = 1.5; CI = 0.9 – 2.5 and OR = 2.6; CI = 1.5 – 4.4) when compared to individuals with moderate CKD (eGFR = 45 – 59 ml/min/73m2)(8). Similarly, in the Heart Estrogen/Progestin Replacement study (n = 1015, age > 80) women with an eGFR <30ml/min/73m2 were independently five times more likely to experience global cognitive impairment measured by the 3MS than women with an eGFR ≥60ml/min/1.73m2 (5). In the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study (6), 23,405 participants with a mean age of 64.9 (SD = 9.6), CKD was independently associated with higher rates of cognitive impairment using the 6-Item Screener (OR = 1.23; 95% CI = 1.06-1.43). Further, for every 10mL/min/1/73m2 decrease in eGFR in participants with CKD, there was an 11% increased prevalence of cognitive impairment (95%CI = 1.04 – 1.19).
Longitudinally, research shows similar results. In the Intervention Project on Cerebrovascular Diseases and Dementia in the Community of Ebersberg (INVADE, n = 3679) (14), the authors compared the association between renal function and cognition by using the Cockcroft-Gault equation to estimate eGFR and the 6-Item Screener to assess cognitive impairment over a 2 year follow-up. Results showed that moderate to severe kidney disease at baseline is independently associated with developing cognitive impairment after a 2-year follow-up (OR = 2.14, 95%CI = 1.18- 3.87)(14). In the Rush Memory and Aging Project, 886 participants were followed up for an average of 3.4 years; eGFR <60ml/min/1.73m2 at baseline was not associated with global cognition (MMSE); however longitudinally, global cognition declined at a faster rate(15). In the Northern Manhattan Study, Khatri et al. (16) only administered the Modified Telephone Interview for Cognitive Status (TICS) on 2,172 participants. They used three measures of kidney function; the Cockcroft-Gault creatinine clearance, the MDRD formula for eGFR, and serum creatinine. They found that individuals with a creatinine clearance of less than 60ml/min, and those between 60 and 90 ml/min declined by an average of 0.4 and 0.2 points on the TICS when compared to individuals with a creatinine clearance over 90ml/min over a mean follow-up of 2.9 years. They also found similar levels of decline in the TICS when using the eGFR and serum creatinine.
By using a quick test that gives an assessment of global or overall cognition, these studies clearly indicate that cognitive function is affected during the course of CKD, with more severe stages or declining eGFR showing associations with poorer scores on cognitive tests. However, despite the convenience of administering a quick and easy test to measure global cognitive function, such as the MMSE (2, 17), the 3MS (3) and the 6-Item-Screener (6), a major limitation is that these tests are only able to indicate impairment on a global cognitive level, which is unspecific and non-informative to health providers who may want to direct care towards specific areas that need attention. Studies that have used tests that measure specific cognitive functions have also shown that some domains of cognition seem to be affected more than others in the course of CKD (4, 8, 18-20).
3.2 Frontal executive function and episodic memory
The majority of studies in both patient populations and in community-dwelling older adults show an association between CKD and/or ESRD and executive dysfunction (4, 8, 13, 19-21). In patient populations, executive dysfunction seems to be present throughout the whole course of moderate (Stage 3) to advanced (Stage 4) CKD and ESRD (Stage 5), with prevalence rates increasing throughout the disease stages (4, 8). One study showed that executive dysfunction is present in 5% of patients with moderate CKD, in 23% of patients with advanced CKD and up to 37% in patients with ESRD (4), and another study found that executive dysfunction is present in 25% of patients with CKD and 25% with ESRD (19). 160 participants with CKD stages 3, 4 and 5 from nephrology and dialysis practices showed a graded association between kidney disease severity and cognitive function, where poorer kidney function was associated with poorer cognitive impairment (p < 0.001) as measured by the 3MS, as well as the Trail Making Tests A and B, and the California verbal learning task (4). Attention and executive function in one study were also found to be more severe in CKD Stage 5 (7). Similar results displaying an association between executive dysfunction and poor renal function were found in community-dwelling populations independent of age, ethnicity, education, vascular and lifestyle variables (3, 13, 18, 22). In a study on a sample of 649 community-dwelling participants (70+ year olds) from the Einstein Aging Study, low eGFR (<45mL/min/1.73m2) was associated with reduced performance on components of frontal executive function, but not with episodic memory or general ability (22). Moderate eGFR (45-59mL/min/1.73m2) and high eGFR (≥60mL/min/m2) were not associated with cognitive function. In the Health and Aging Brain among Latino Elders study (23), 437 Mexican Americans with low eGFR (<45 mL/min/1.73m2) showed poorer global cognitive function as well as poorer processing speed, executive function, and visuospatial skills than individuals with moderate (45-59 mL/min/1.73m2) or high (≥60 mL/min/1.73m2) eGFR. In another study, Event Related Potentials (ERPs) were used to measure speed of cognitive processing in individuals with CKD and ESRD; the authors found significant prolongation of P3 latencies (corresponding to slower processing speed) in CKD stages 4 and 5 (17).
Cognitive impairment in CKD seems to also extend to memory in some cases, (4, 5, 7, 19, 20) but not in others (8, 18). One study shows that up to 15% of CKD patients and 13% of patients on hemodialysis have memory impairment (19); another one found that up to 24% of individuals with CKD receiving hemodialysis treatment have memory impairment (7). It is unclear though at what stage memory becomes more impaired; although some authors suggest that memory impairment takes place in more advanced CKD (19, 24). For example, in 923 community-based individuals over 60 years, participants with eGFR <60mL/min/1.73m2 did not show any significant differences on episodic memory tasks than participants with eGFR ≥60 mL/min/1.73m2; however, significant differences (p < .01) were present for a Global composite, and Visual Spatial Organization, and Scanning and Tracking, which measure executive function (18). In an age- and education- matched case-controlled study, on 51 persons with CKD and 55 without the disease, significant differences on executive function (Mental set Shifting and Inhibition; p < .05 ) as well as memory measures (California Verbal Learning Task-II; p < .001) were found between the groups with up to 46% of individuals with CKD showing significant impairments in verbal memory. The Chronic Renal Insufficiency Cohort (CRIC) (mentioned above) also applied the Trail Making Tests A and B to measure executive function and Boston Naming to measure fluency, all of which were also significantly different amongst groups with the advanced CKD and the ESRD groups showing poorer performance than the moderate CKD group; however Category Fluency and Immediate and Delayed Recall were not significant (8).
Although longitudinal research is sparse in this area, the Baltimore Longitudinal Study of Aging followed up 2,116 participants who had no dementia or previous stoke at baseline, every two years. Renal function was assessed using eGFR and the inverse of serum creatinine (1/SCr), while for cognition the California Verbal Learning Test (CVLT) was used to assess verbal memory, the Benton Visual Retention Test (BVRT) to assess visual memory, Category and Letter fluency, Trail Making Tests A and B, the Boston Naming Test, and Digit Span. The only significant associations (p < .001) between increased creatinine concentrations/ low eGFR and longitudinal cognitive decline were in the CVLT and the BVRT, which are both memory based tests. The Rush Memory and Aging Project also showed that low eGFR (<60ml/min/1.73m2) was associated with faster decline in episodic memory, semantic memory, and working memory, but not with perceptual speed or visuospatial abilities. At cross-section none of these variables were significant (15).
3.3 MCI and dementia in chronic kidney disease
Cognitive impairment in CKD patients seems to vary according to CKD stage thus suggesting casual associations between the two mechanisms (3). However, few studies explored the association between renal function and dementia (2, 25, 26), and only three investigated the association between CKD/ESRD and MCI (12, 19).
In CKD, vascular dementia seems to feature more prominently than other dementias. In the Three-City (3C) Study the incidence of dementia all causes and dementia of a vascular type was investigated in 9,294 participants over the age of 60 (2). Results showed that an initial low eGFR was not associated with increased risk of developing dementia; however, decline in eGFR was associated with all cause dementia, and vascular dementia, but not with Alzheimer's disease; an annual decline of 4ml/min/1.73m2 carried a relative risk of 1.69 (CI = 1.05 – 2.73) for dementia all causes, and increased relative risk of 5.35 (CI = 1.76 – 16.32) for vascular dementia. Similar results were found in the Cardiovascular Health Cognition Study where 3,349 participants over the age of 65 were evaluated for risk of dementia and renal function. In this study renal impairment was explored using serum creatinine (1/SCr), with cutoff values of ≥1.3 mg/dl for women and ≥ 1.5 mg/dl for men (27). For every 0.5-unit decrease in 1/SCr (this is equivalent to an increase of 2.0 SCr) the authors found a 26% increased risk of incident dementia (HR = 1.26; 95% CI = 1.02 – 1.54), independent of age, sex, ethnicity, weight, level of education, smoking, apoE genotype, diabetes, hypertension, and chronic heart disease. Studied categorically (i.e. high vs low SCr), high SCr was associated with a 37% increased risk of incident dementia (HR = 1.37, 95% CI = 1.06 – 1.78), after controlling for the same covariates. For vascular-type dementia alone, the risk was even higher for individuals with high SCr (HR = 1.58, 95% CI = 1.10 – 2.26). The association did not hold for Alzheimer-type dementia (27). Furthermore, in a study investigating psychiatric disorders among 508 patients with a mean age of 65.7 years (SD = 14.4 years) with ESRD, the authors (25) found that dementia of the vascular type was the most common disorder in ESRD second only to delirium. Compared to the general population the 1-year incidence rate of Alzheimer's dementia was similar to this patient group (1%); however, the 1-year incidence rate of multi-infarct dementia was 3.4%, making it 7.4 times larger in their patient-group than in the general population.
Although cognitive impairment is well studied in CKD, only three studies (12, 19, 23) to our knowledge have assessed MCI as an outcome. In their study, Post et al. (19) studied 24 patients with CKD and 27 patients with ESRD receiving hemodialysis (HD) without any history of dementia, stroke or neurodegenerative disease. They defined aMCI as ≥1.5 SDs below age-and education-matched norms on long and short delayed memory tests. naMCI was defined as ≥1.5 SDs below the norm on two or more tests in a non-memory domain, such as executive function, language and/or attention. Post et al.'s (19) study on the cognitive profile of patients with CKD and ESRD on HD, results showed that ESRD patients had a higher prevalence of MCI than CKD patients (89% vs. 63%), and that up to 70% of MCI patients could be classified as naMCI. The authors also found non-memory deficits even in patients classified as aMCI. Further, in most cases individuals showed impairment in more than one domain of non-memory based function, suggesting an increased risk of developing vascular dementia. Compared to CKD patients, ESRD patients on HD did not differ significantly on memory measures, but they had significantly lower scores on tests measuring processing speed, language, and attention, further suggestive of changes associated with vascular dementia. These results further strengthen previous findings on associations between executive function, vascular dementia and CKD. In the Einstein Aging Study, 10.5% of the 622 participants that participated in the study had low eGFR (<45 mL/min/1.73m2); low eGFR was independently associated with MCI of the dysexecutive (OR = 4.18, 95%CI =1.67 – 10.44) and amnestic (OR = 30.4, 95%CI = 1.10 – 8.33) type (12).
More recently, research is moving forward to predicting which CKD participants will develop MCI based on blood samples; in the Health and Aging Brain among Latino Elders study (23), a blood-based profile that accurately predicted CKD-related MCI within 12 months of follow-up in individuals with eGFR <60ml mL/min/1.73m2. This is a move forward from estimating prevalence rates within the CKD spectrum. Despite the few studies, results have been consistent in reporting increased prevalence of MCI and dementia in individuals with CKD. Learning what mechanisms underlie the kidneys and cognition may also help in not only accurately predicting future MCI and dementia, but knowing what to treat to avoid them.
4. Cognitive impairment in albuminuria
Studies that assess the association between CKD and cognition mostly use creatinine or eGFR as a marker of kidney function. Fewer studies assessed the relationship between cognition and albuminuria. Albuminuria is the excessive loss of protein in urine, and a marker of kidney damage, amongst other underlying conditions that include cumulative vascular damage related to hypertension, diabetes, and inflammation (28-30). It is known to be an early indicator of CKD (31).
Prevalence of albuminuria increases with kidney impairment; it is estimated that 24% of individuals with CKD stage 3 have albuminuria (1). Individuals with albuminuria have an increased risk of further accelerated eGFR loss when compared to controls (32). Higher levels of albuminuria have been associated with increased mortality (33) and cognitive impairment in older adults (28, 34-38). Studies have explored whether albuminuria and cognition are associated at the cross-sectional (28, 34, 37, 38) and longitudinal level (36, 39, 40); however, concordance among eGFR, albumuninuria, and cognitive impairment is not as clear and results at both cross-sectional levels and longitudinally are mixed.
At cross-section, Brazilay et al. (28) reported that the presence of albuminuria was associated with a 1.22 odds (95%CI = 1.15 - 1.29) of increased risk for dementia in 2,316 adults at 65 years or older enrolled in the Cardiovascular Health Study; after adjustments for demographics, ApoE4, cardiovascular disease and associated risk factors, this association became attenuated but was still statistically significant (OR = 1.13, 95%CI = 1.04 – 1.23). The results remained significant when albuminuria was analyzed categorically (<30mg/g vs ≥30mg/g; OR = 1.58, 95%CI = 1.09 – 2.30). As a smaller subset analysis, the authors also looked at incident dementia as an outcome; results also showed that albuminuria predicted incident dementia without (OR = 1.22, 95%CI = 1.15 – 1.29) and with adjustments for demographics (OR = 1.13, 95%CI = 1.04 – 1.23). Studying the dementia subtypes, strong associations were found between doubling of albuminuria and incident vascular dementia (OR = 1.17, 95%CI = 1.12 – 1.23), and borderline associations were found for incident Alzheimer's disease (OR = 1.10, 95%CI = 0.98 – 1.22). In a community study investigating different domains of cognition in association to albuminuria among 335 older homebound adults with a mean age of 73.4, results showed that albuminuria was independently associated with executive dysfunction (p < .05) but not with memory or working memory (41). This result was not found in the National Health and Nutrition Examination Survey of 1999-2002 where Vupputuri et al. (34) did not find an association between cognitive function, as measured by Digit Symbol Substitution Test and albuminuria in healthy older adults; however, an association was present between albuminuria and the low (OR = 6.5, 95%CI = 2.0 – 20.5) and middle (OR = 3.5, 95%CI = 1.2 – 10.3) tertiles of cognitive function in individuals with peripheral artery disease independent of cerebrovascular and inflammation variables. Another study in the National Health and Nutrition Examination Survey of 1999-2002, Kuo et al. (37) found an overall association between the Digit Symbol Substitution Test and albuminuria; however, this association was absent when they excluded individuals with peripheral artery disease, suggesting that underlying vascular mechanisms drive this association.
Longitudinal studies also report associations between albuminuria and cognitive decline with mixed results. Barzilay et al. (36) found that at baseline, 28,384 participants with vascular disease or diabetes with microalbuminuria and macroalbuminuria were at higher odds (ORs = 1.26, 95%CI = 1.11 – 1.44 and OR = 1.21 95%CI = 0.94 – 1.55) of cognitive decline defined as MMSE drop of 3 points over a 5-year follow up than those without albuminuria. Jassal et al. (40) reported longitudinal associations between baseline albumin/creatinine ratio of ≥30mg/g and cognitive decline after 6.6 years of follow-up as measured by the MMSE and Category Fluency in men, but failed to find this association in women or at cross-section. Meanwhile in Sajjad et al. (39) there were no significant differences in cognitive decline in participants with an eGFR <60 ml/min per 1.73 m2compared with eGFR ≥60 ml/min per 1.73 m2 in either age-adjusted or multivariable models; however, significantly worse decline in cognitive function was seen in participants with an Albumin to Creatinin Ratio (ACR) ≥5 mg/g compared with those with an ACR <5 mg/g independent of blood pressure and insulin. Kurella et al. (42) reported greater risk for incident cognitive impairment (a score of ≤4 on the Six-Item Screener) in individuals with ARC 30 – 300mg/g (OR= 1.31, 95%CI = 1.12 – 1.55) and in individuals with ACR ≥ 300mg/g (OR = 1.57, 95%CI = 1.15 – 2.14) compared with individuals with ACR < 10mg/g. This association was not found when comparing individuals with eGFR <60ml/min/1.73m2 to those with ≥60ml/min/1.73m2 or higher. Once they stratified by ACR into groups of <10, 10-29, 30-229, and ≥300mg/g, results showed that in participants with ACR <10mg/g and with eGFR <60 ml/min/1.73m2 the risk for incident cognitive impairment was at an odds of 1.30 (95%CI = 1.02 – 1.66) when compared to those with eGFR ≥60 ml/min/1.73m2. Results were attenuated when the higher ACRs were analyzed, indicating that elevated ACR and eGFR are complementary risk factors for cognitive decline. Lastly, in 205 participants with diabetes with a mean age of 75.3 years, Bruce et al. (43) found that ACR predicted cognitive decline (defined as change in the Clinical Dementia Rating Scale) (OR = 1.37, 95%CI = 1.05 – 1.78) independent of age and education. Joosten et al. (38) investigated the association between cognitive function and albuminuria across a broad age range (35 – 82 years) of 4095 community-dwelling participants from the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study. Their results showed that presence of albuminuria (≥30 mg/24h), but not eGFR, was associated with poorer scores on the Ruff Figural Fluency Test in the youngest tertile (age range = 34 – 48; B = 5.3, 95%CI = 0.6 – 9.2) but not in the oldest tertiles (age ranges = 49 = 59 and 60 – 82; B = −1.76, 95%CI = −5.3 – 1.9 and B = −0.50, 95%CI = −3.1 – 2.1). When excluding individuals with history of cardiovascular disease, cerebrovascular disease, diabetes mellitus, or an eGFR of < 60 ml/min/1.73m2, the associations remained significant ((B = −10.6, 95%CI = −19.9 - −1.3).
5. Potential mechanisms explaining the association between kidney and cognitive function
CKD is associated with vascular pathology; however, it remains unclear what causes cognitive impairment in CKD. Overall, there is greater prevalence of cerebrovascular disease (CVD) and traditional CVD risk factors, including hypertension, diabetes, stoke and myocardial infarction, in individuals with CKD (44), all of which are associated with cognitive impairment and dementia. Furthermore, since cognitive impairment in CKD predominantly takes places in the non-memory domains, researchers hypothesize on potential vascular etiologies.
A lot of speculation on the association between CKD and cognitive impairment revolves around theories on underlying common mechanisms that may mediate the kidney-cognition association. Non-traditional vascular risk factors such as, anemia, oxidative stress, elevated inflammatory cytokines as well as changes in lipid and homocystine levels present in CKD are thought to affect cognitive function (9, 45-48). Putative neurotoxins including parathyroid hormone and nitrogen metabolism have also been mentioned as possible mediating factors in cognitive impairment and CKD (3). Imaging studies that demonstrate elevated white matter hyperintensities in individuals with both cognitive impairment (49) and low eGFR(16, 50) or albuminuria (51, 52) support the hypothesis that small-vessel pathology is a result of endothelial dysfunction, which is present in both dementia and CKD, as a key player in the impairment. Endothelial dysfunction has been demonstrated in the early stages of renal disease and in individuals with CKD risk factors (53, 54). Other studies suggest that the kidney could be reflecting early vascular damage, for example small-vessel damage (2). Cerebrovascular damage such as white-matter disease and subcortical strokes have also been suggested to explain executive dysfunction in CKD due to the association found between kidney disease and subclinical brain infarcts (26) that may arise from disruptions in frontal-subcortical circuits (4). Studies on the association between albuminuria and cognitive impairment have found stronger associations with vascular dementia (28), which is suggestive of underlying roles of vascular mechanisms because albuminuria is associated with endothelial damage and vascular disease. It also points out that microvascular disease in one organ may be reflective of microvascular disease in another organ in individuals with macrovascular disease (36).
Most studies however, also show that the association between cognitive impairment and CKD persists after adjusting for vascular risk factors, and inflammation (3, 4, 8, 53-55). This implies that other factors are involved in the occurrence of cognitive impairment in CKD. We illustrate hypothesized mechanisms underlying the association between kidney function and cognition in Figure 1. The various explanations and speculations demonstrate that research is still wanting of a casual association explaining what connects the brain and the kidneys. New initiatives, such as the Brain in Kidney Disease (BRINK) cohort study aim to define the mechanisms that contribute to increased risk for cognitive impairment in CKD (56).
Figure 1.
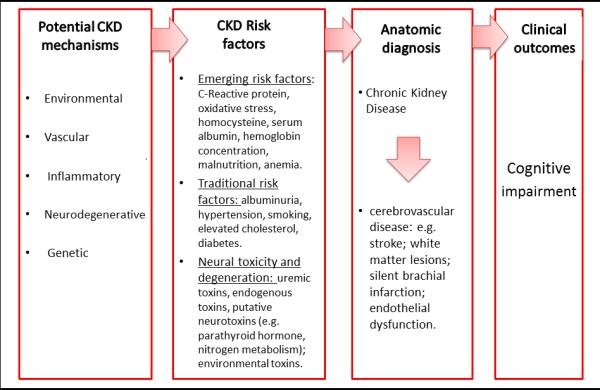
Figure depicting potential mechanisms underlying causes of chronic kidney disease and cognitive impairment.
6. Future directions
There is a lot of room for research to expand on in this field. Firstly, longitudinal studies exploring declining renal and cognitive function are needed to start strengthening the association between these two mechanisms, and potentially start inferring causality. The use of mediation and moderator analyses may also be useful in this context to discover pathways between these two mechanisms. Secondly, neurocognitive testing suggests that frontal executive functioning is particularly sensitive to the effects of kidney disease (4); however, structural brain imaging is lacking to verify the hypothesis that subcortical deficits are present. Imaging studies will clarify the etiology of cognitive impairment and underlying mechanisms in the brain-kidney association; it will also highlight the areas that are most affected, and follow any developments of white matter hyperintensities during the course of CKD. Lastly, although the association between kidney disease and cognitive impairment is evident, screening methods in CKD patients still seem to be unavailable. It would be helpful to gather enough evidence for the appropriate tools and the specific cut-off points that would determine when help is required (8). If executive function is followed by memory impairment along the CKD course, multiple screening processes may need to take place. Executive dysfunction will hinder any patient to plan ahead, organize tasks, maintain attention, follow directions and carry out ADLs independently including adhering to treatment regiments. Thus, the importance of screening and treating early is essential to prevent further avoidable decline.
7. Conclusion
Most domains of cognitive function seem to be affected by poor renal function across all stages of CKD. Executive function is the most affected cognitive domain, and the most prevalent dementia that emerges during the course of CKD is the vascular subtype. Although there are a number of theories attempting to explain this association, it is not yet clear what underlies it. Determining the casual and /or mediating factors will help target at-risk populations earlier, intervene sooner, and possibly prevent cognitive decline. Longitudinal research, brain imaging, and better screening tools may help in developing a better understanding of casual factors underlying the association between these two mechanisms, and consequently develop better screening and treatment tools.
Acknowledgments and funding sources
We would like to gratefully acknowledge the funding sources that supported this research: The Einstein Aging Study (PO1 AG03949) from the National Institutes on Aging program; the National Institutes of Health CTSA (1UL1TR001073) from the National Center for Advancing Translational Sciences (NCATS), the Sylvia and Leonard Marx Foundation, and the Czap Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: All authors declare that there are no financial, personal, or other potential conflicts of interest.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. PRevalence of chronic kidney disease in the united states. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Helmer C, Stengel B, Metzger M, et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology. 2011;77:2043–2051. doi: 10.1212/WNL.0b013e31823b4765. [DOI] [PubMed] [Google Scholar]
- 3.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 4.Kurella M, Chertow GM, Luan J, et al. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 5.Kurella M, Yaffe K, Shlipak MG, et al. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Roman S, Ostrosky-Solis F, Morales-Buenrostro LE, et al. Neurocognitive profile of an adult sample with chronic kidney disease. Journal of the International Neuropsychological Society : JINS. 2011;17:80–90. doi: 10.1017/S1355617710001219. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Ackerson L, Kurella Tamura M, et al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2010;58:338–345. doi: 10.1111/j.1532-5415.2009.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308:2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zammit AR, Katz MJ, Zimmerman ME, et al. Low eGFR is associated with dysexecutive and amnestic mild cognitive impairment. Alzheimers Dement (Amst) 2015;1:152–159. doi: 10.1016/j.dadm.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc. 2008;56:2082–2088. doi: 10.1111/j.1532-5415.2008.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etgen T, Sander D, Chonchol M, et al. Chronic kidney disease is associated with incident cognitive impairment in the elderly: the INVADE study. Nephrol Dial Transplant. 2009;24:3144–3150. doi: 10.1093/ndt/gfp230. [DOI] [PubMed] [Google Scholar]
- 15.Buchman AS, Tanne D, Boyle PA, et al. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009;73:920–927. doi: 10.1212/WNL.0b013e3181b72629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke. 2007;38:3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madan P, Kalra OP, Agarwal S, et al. Cognitive impairment in chronic kidney disease. Nephrol Dial Transplant. 2007;22:440–444. doi: 10.1093/ndt/gfl572. [DOI] [PubMed] [Google Scholar]
- 18.Elias MF. Chronic kidney disease, creatinine and cognitive functioning. Nephrology, dialysis, transplantation. 2009;24:2446–2452. doi: 10.1093/ndt/gfp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Post JB, Jegede AB, Morin K, et al. Cognitive profile of chronic kidney disease and hemodialysis patients without dementia. Nephron Clin Pract. 2010;116:c247–255. doi: 10.1159/000317206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornton WL, Shapiro RJ, Deria S, et al. Differential impact of age on verbal memory and executive functioning in chronic kidney disease. Journal of the International Neuropsychological Society : JINS. 2007;13:344–353. doi: 10.1017/S1355617707070361. [DOI] [PubMed] [Google Scholar]
- 21.Hailpern SM, Melamed ML, Cohen HW, et al. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol. 2007;18:2205–2213. doi: 10.1681/ASN.2006101165. [DOI] [PubMed] [Google Scholar]
- 22.Zammit AR, Katz MJ, Lai JY, et al. Association between renal function and cognitive ability domains in the einstein aging study: a cross-sectional analysis. J Gerontol A Biol Sci Med Sci. 2015;70:764–770. doi: 10.1093/gerona/glu185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szerlip HM, Edwards ML, Williams BJ, et al. Association Between Cognitive Impairment and Chronic Kidney Disease in Mexican Americans. J Am Geriatr Soc. 2015;63:2023–2028. doi: 10.1111/jgs.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griva K, Thompson D, Jayasena D, et al. Cognitive functioning pre- to post-kidney transplantation--a prospective study. Nephrol Dial Transplant. 2006;21:3275–3282. doi: 10.1093/ndt/gfl385. [DOI] [PubMed] [Google Scholar]
- 25.Fukunishi I, Kitaoka T, Shirai T, et al. Psychiatric disorders among patients undergoing hemodialysis therapy. Nephron. 2002;91:344–347. doi: 10.1159/000058418. [DOI] [PubMed] [Google Scholar]
- 26.Seliger SL, Longstreth WT, Jr., Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16:3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 27.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15:1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 28.Barzilay JI, Fitzpatrick AL, Luchsinger J, et al. Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis. 2008;52:216–226. doi: 10.1053/j.ajkd.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried L. Albuminuria and cognitive impairment. Clin J Am Soc Nephrol. 2012;7:376–378. doi: 10.2215/CJN.00210112. [DOI] [PubMed] [Google Scholar]
- 30.Barzilay JI, Peterson D, Cushman M, et al. The relationship of cardiovascular risk factors to microalbuminuria in older adults with or without diabetes mellitus or hypertension: the cardiovascular health study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;44:25–34. doi: 10.1053/j.ajkd.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Verhave JC, Gansevoort RT, Hillege HL, et al. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney international. Supplement. 2004:S18–21. doi: 10.1111/j.1523-1755.2004.09205.x. [DOI] [PubMed] [Google Scholar]
- 32.Halbesma N, Kuiken D-S, Brantsma AH, et al. Macroalbuminuria Is a Better Risk Marker than Low Estimated GFR to Identify Individuals at Risk for Accelerated GFR Loss in Population Screening. Journal of the American Society of Nephrology. 2006;17:2582–2590. doi: 10.1681/ASN.2005121352. [DOI] [PubMed] [Google Scholar]
- 33.O'Hare AM, Hailpern SM, Pavkov ME, et al. Prognostic implications of the urinary albumin to creatinine ratio in veterans of different ages with diabetes. Archives of internal medicine. 2010;170:930–936. doi: 10.1001/archinternmed.2010.129. [DOI] [PubMed] [Google Scholar]
- 34.Vupputuri S, Shoham DA, Hogan SL, et al. Microalbuminuria, peripheral artery disease, and cognitive function. Kidney international. 2008;73:341–346. doi: 10.1038/sj.ki.5002672. [DOI] [PubMed] [Google Scholar]
- 35.Fried L. Albuminuria and Cognitive Impairment. Clinical Journal of the American Society of Nephrology. 2012;7:376–378. doi: 10.2215/CJN.00210112. [DOI] [PubMed] [Google Scholar]
- 36.Barzilay JI, Gao P, O'Donnell M, et al. Albuminuria and decline in cognitive function: The ONTARGET/TRANSCEND studies. Archives of internal medicine. 2011;171:142–150. doi: 10.1001/archinternmed.2010.502. [DOI] [PubMed] [Google Scholar]
- 37.Kuo HK, Lin LY, Yu YH. Microalbuminuria is a negative correlate for cognitive function in older adults with peripheral arterial disease: results from the U.S. National Health and Nutrition Examination Survey 1999-2002. Journal of internal medicine. 2007;262:562–570. doi: 10.1111/j.1365-2796.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- 38.Joosten H, Izaks GJ, Slaets JP, et al. Association of cognitive function with albuminuria and eGFR in the general population. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:1400–1409. doi: 10.2215/CJN.05530610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sajjad I, Grodstein F, Kang JH, et al. Kidney Dysfunction and Cognitive Decline in Women. Clinical Journal of the American Society of Nephrology : CJASN. 2012;7:437–443. doi: 10.2215/CJN.05330611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am J Epidemiol. 2010;171:277–286. doi: 10.1093/aje/kwp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53:438–447. doi: 10.1053/j.ajkd.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurella Tamura M, Muntner P, Wadley V, et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58:756–763. doi: 10.1053/j.ajkd.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruce DG, Davis WA, Casey GP, et al. Predictors of cognitive decline in older individuals with diabetes. Diabetes care. 2008;31:2103–2107. doi: 10.2337/dc08-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 45.Abramson JL, Jurkovitz CT, Vaccarino V, et al. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int. 2003;64:610–615. doi: 10.1046/j.1523-1755.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 46.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–334. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. The New England journal of medicine. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 48.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 49.Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Archives of neurology. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikram MA, Vernooij MW, Hofman A, et al. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39:55–61. doi: 10.1161/STROKEAHA.107.493494. [DOI] [PubMed] [Google Scholar]
- 51.Knopman DS, Mosley TH, Jr., Bailey KR, et al. Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci. 2008;271:53–60. doi: 10.1016/j.jns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wada M, Nagasawa H, Kurita K, et al. Microalbuminuria is a risk factor for cerebral small vessel disease in community-based elderly subjects. J Neurol Sci. 2007;255:27–34. doi: 10.1016/j.jns.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 53.Stam F, van Guldener C, Becker A, et al. Endothelial dysfunction contributes to renal function-associated cardiovascular mortality in a population with mild renal insufficiency: the Hoorn study. J Am Soc Nephrol. 2006;17:537–545. doi: 10.1681/ASN.2005080834. [DOI] [PubMed] [Google Scholar]
- 54.Stam F, van Guldener C, Schalkwijk CG, et al. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant. 2003;18:892–898. doi: 10.1093/ndt/gfg080. [DOI] [PubMed] [Google Scholar]
- 55.Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial. 2008;21:29–37. doi: 10.1111/j.1525-139X.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 56.Murray AM, Bell EJ, Tupper DE, et al. The Brain in Kidney Disease (BRINK) Cohort Study: Design and Baseline Cognitive Function. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


