Abstract
Alcohol-use disorders (AUD) persist in the United States and are heavily associated with an increased susceptibility to respiratory viral infections. Respiratory syncytial virus (RSV) in particular has received attention as a viral pathogen commonly detected in children and immune-compromised populations (elderly, asthmatics), yet more recently was recognized as an important viral pathogen in young adults. Our study evaluated the exacerbation of RSV-associated illness in mice that chronically consumed alcohol for 6 weeks prior to infection. Prior studies showed that lung viral titers remained elevated in these animals, leading to a hypothesis that T-cell activation and immune specificity were deficient in controlling viral spread and replication in the lungs. Herein, we confirm a reduction in RSV-specific IFNγ production by CD8 T cells and a depolarization of Th1 (CD4+IFNγ+) and Th2 (CD4+IL-4+) T cells at day 5 after RSV infection. Furthermore, over the course of viral infection (day 1 to day 7 after RSV infection), we detected a delayed influx of neutrophils, monocytes/macrophages, and lymphocytes into the lungs. Taken together, the data show that both the early and late adaptive immunity to RSV infection are altered by chronic ethanol consumption. Future studies will determine the interactions between the innate and adaptive immune systems to delineate therapeutic targets for individuals with AUD often hospitalized by respiratory infection.
Keywords: RSV – respiratory syncytial virus, AUD – alcohol-use disorder, Th1 – Type 1 CD4+IFNγ+ T-helper cell, Th2 – Type 2 CD4+IL-4+ T-helper cell, APC – antigen-presenting cell
Introduction
Nearly 18 million individuals in the United States abuse alcohol (Grant, Dawson, et al., 2004; Grant, Stinson, et al., 2004; NIAAA, 2000). The detrimental effects of alcohol use on organs involved predominantly in alcohol metabolism (e.g., liver) are well-described (Lieber, 1971; Rubin & Lieber, 1971). More recently, however, interest has risen in how alcohol use and metabolism alter lung airway function and immunity (Berger et al., 2014; Oldenburg, Poole, & Sisson, 2012; Simet et al., 2012; Skerrett, Niederman, & Fein, 1989; Yang et al., 2015). Alcohol-use disorders (AUD) are associated with an increased risk of bacterial respiratory infections, including M. tuberculosis and S. pneumoniae (Al-Sanouri, Dikin, & Soubani, 2005; Simet & Sisson, 2015). According to the Centers for Disease Control and Prevention, individuals that chronically abuse alcohol have a 10 times greater risk of acquiring bacterial pneumonia, and these individuals are four times more likely to die due to complications associated with bacterial pneumonia than the non-drinker (CDC, 2004). Lung viral infections, specifically, make the lung permissive to opportunistic secondary bacterial pathogens such as S. pneumoniae (Vander Top, Wyatt, & Gentry-Nielsen, 2005) or P. carinii (Gamble, Mason, & Nelson, 2006; Shellito, 1998; Shellito & Olariu, 1998). Understanding alcohol-induced alterations in the immune response to these viral infections is key to preventing secondary infections and developing therapies for at-risk individuals likely to contract respiratory infections.
Chronic alcohol consumption is associated with decreased viral clearance, increased pulmonary inflammation, and a modified anti-viral interferon response in animal studies (Jerrells et al., 2007). Namely, prior studies show that the anti-viral CD8+IFNγ+ T-lymphocyte population is not properly activated in the setting of chronic alcohol consumption and influenza A viral infection. Subsequently, this T-cell defect significantly delays lung viral clearance (Meyerholz et al., 2008). BALB/c mice effectively clear a respiratory syncytial virus (RSV) infection within 5 days of exposure (Hashimoto et al., 2009; Muñoz, McCarthy, Clark, & Hall, 1991; Topham, Tripp, & Doherty, 1997; Varga & Braciale, 2002); however, this requires a properly coordinated CD4+ and CD8+ T-cell response (Cannon, Openshaw, & Askonas, 1988; Varga, Wang, Welsh, & Braciale, 2001). The CD8+ T-cell response is activated through an interaction with antigen-presenting cells (APC) and the type 1 CD4+ T-helper (Th1) cells (i.e., CD4+IFNγ+ T cells). These interactions between APC and T-helper cells are necessary to activate the specific anti-viral CD8+ T-mediated immune defense (Doherty et al., 1997; Topham et al, 1997; Zhong, Roberts, & Woodland, 2001). RSV has a unique immune signature in the lungs; RSV is the only respiratory viral pathogen known to skew the anti-viral T-cell response toward a type 2, CD4+ T-helper (Th2, CD4+ IL-4+) response as opposed to a Th1 response (Christiaansen, Knudson, Weiss, & Varga, 2014; Lotz & Peebles, 2012). The Th2 cell responses are associated with allergic or anti-parasitic responses. Moreover, the Th2 skewing induced during an RSV infection is commonly associated with the severity of respiratory symptoms including bronchiolitis, excessive mucus production, and barking cough noted in infected infants and children (Christiaansen et al., 2014; Peebles & Moore, 2007). The aim of this study was to evaluate CD4+ and CD8+ T-cell responses following alcohol consumption and RSV infection, with the intention of understanding the interplay of alcohol on anti-viral immunity in the lung. We hypothesized that CD4 and CD8 T-cell activation and cytokine (IFNγ, IL-4) production would be attenuated by chronic alcohol consumption in RSV-infected mice. This study provides evidence that chronic alcohol consumption substantially affects anti-viral immunity causing increased RSV titers, decreased CD8+IFNγ activation, and decreased CD4+IL-4 responses that impact immune cell migration and activation in the lung.
Materials and methods
Mice and chronic alcohol feeding
Female BALB/c mice, between 6–8 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME). Mice acclimated for a minimum of 1 week prior to starting the experimental protocol. Animals were randomly assigned to ethanol feeding or water feeding as control. We utilized the Cook model of ethanol feeding (Cook et al., 2004, 2007), whereby mice acclimated to ethanol feeding by gradually increasing the volume of ethanol in drinking water over a 1-week period (10% ethanol [w/v] in sterile water for 2–3 days, followed by 15% [w/v] in sterile water for two additional days, followed by 18% ethanol [w/v] for the remainder of the experiment) (Elliott, Sisson, & Wyatt, 2007). No significant weight loss was observed due to alcohol feeding. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In addition, animal experiments were completed with the approval and oversight of the University of Nebraska Medical Center's Institutional Animal Care and Use Committee.
RSV infection
Mice from ethanol-feeding and control-feeding groups were randomly chosen to receive an RSV infection. Mice were lightly anesthetized with isoflurane and intranasally instilled with RSV (Advanced Biotechnologies, Eldersburg, MD) diluted in 50 μL of sterile saline, at 3,250 TCID50 U per mouse. Mock-infected mice were similarly anesthetized and instilled with saline as vehicle control. Body weight was monitored to assure that no more than 20% of initial starting body weight was lost during the infection period. Subsets of mice were euthanized at days 1, 3, 5, and 7 post-infection.
TCID50/mL method – viral infectivity assay
Murine lungs were weighed and homogenized in base Earl's Minimum Essential Medium (EMEM) (ATCC, Manassas, VA) for the viral infectivity assay. Briefly, human epithelial type 2 (HEp-2) cells (ATCC) were sub-cultured into 96-well plates (Corning, Pittsburgh, PA) at a rate of 5 × 105 cells per well in EMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Life Technologies, Grand Island, NY). Upon reaching 85–90% confluence, cell layers were washed with phosphate-buffered saline (PBS), and then inoculated with 10-fold dilutions of mouse lung homogenates. After a 2-h incubation at 37 °C in a 5% CO 2 incubator, the lung homogenates were removed by aspiration. Serum-free base EMEM was added to all the wells, and plates were incubated for up to 7 days at 37 °C, 5% CO 2. Each well was examined for viral infection, and quantitation of virus was determined using the Reed-Munich equation (Warren et al., 2015).
Bronchoalveolar lavage and immune cell detection
Subsets of mice were euthanized at days 5 and 7 post-infection and bronchoalveolar lavage (BAL) fluids collected by washing lungs with 3 × 1-mL saline through a small tracheal incision, previously described (Elliott et al., 2007). Cell pellets were acquired by centrifugation from lung lavage fluids and stained with antibodies developed against extracellular anti-mouse antigens: hamster anti-mouse CD3ε APC-Cy7 (clone: 145-2C11), rat anti-mouse CD4-PerCPCy5.5 (clone 145-2C110), rat anti-mouse CD45-APC (clone: 30-F11) (BD Biosciences, San Jose, CA) and rat anti-mouse CD8β-eFlour450 (clone: eBioH35-17.2) (eBiosciences, San Diego, CA). Cell data acquisition was completed using the BD Biosciences LSRII. Subsequent gating and analysis were performed using Flowjo software (Asland, OR). In separate experiments, BAL cells were prepared onto cytospin slides at a rate of 5 × 105 cells per slide. Slides were stained with Diff-Quik reagent (Fisher Scientific, Houston, TX) and neutrophils, lymphocytes, and monocyte/macrophage were quantified on at least ten 10X fields per slide.
Lung-associated T-cell isolation procedure
Total lung cells were also collected from 5 mice per treatment group. After opening the chest cavity, the right ventricle was infused with 10 mL of sterile heparinized (5 U/mL) PBS to remove blood from the pulmonary vasculature. Lung tissues were dissociated through a 70-uM cell strainer into a petri dish containing RPMI supplemented with type I collagenase (20 U/mL) (Worthington, Lakewood, NJ) (Poole et al., 2012). Type I collagenase was activated by placing the petri dishes in a 37 °C incubator for 15 min. The single-cell suspension of lung cells was filtered a second time through a 30-uM cell strainer into a conical tube and washed once with complete RPMI (10% fetal bovine serum [FBS], 1% penicillin/streptomycin, and 50-μM 2-mercaptoethanol). Lung cells were quantified by hemocytometer and recorded. Viability was assessed and assured using trypan blue exclusion. Next, T cells were isolated using the manufacturer's instructions included with the pan T-cell isolation kit available from Miletyni (San Diego, CA). Post-sort T-cell purification was 92.2% ± 3.99 across all T-cell activation experiments.
T-cell activation assay
Activation and cytokine expression profiles of infiltrating T cells were investigated by fluorescence-activated cell-sorting (FACS) analysis. 1 × 106 lung T cells were stimulated with RSV-specific peptide (M282–90, 1 μM) (Anaspec, Fremont, CA) or PMA/Ionomycin (50 ng/mL and 1 μg/mL, respectively) for 6 h to assess CD8+ T-cell activation and CD4+ T-cell polarization, respectively. Fresh RPMI media without PMA/Ionomycin or M2 peptide was applied to cells as a negative control. Following the stimulation period, brefeldin A (0.5 ng/mL, BD Biosciences) was added to inhibit protein transport for 2 h before extracellular staining with rat anti-mouse CD8β eFlour 450 (clone: eBioH35-17.2) (eBioscience) hamster anti-mouse CD3ε APC-Cy7 (clone: 145-2C11), and rat anti-mouse CD4 PerCP-Cy5.5 (clone: RM4-5) (BD Biosciences). All cells were treated with cytofixation and permeabilization buffer (BD Biosciences) for 20 min prior to staining with rat anti-mouse IL-17A PE (clone: TC11-18H10.1), IFNγ FITC (clone: XMG1.2) and IL-4 APC (clone: 11B11) (BD Biosciences) for 45 min. Cells were washed two times with PBS containing 2% FBS and 0.1% NaN3 and analyzed on the FACS LSRII (BD Biosciences). At least 10,000 CD8+ events and 30,000 CD4+ events were acquired to ensure sufficient detection of intracellular IFNγ, IL-4, and IL-17A.
Statistical methods
Data are representative of two or three separate experiments and presented as mean + SEM. Statistical analysis was conducted using GraphPad Prism Version 6 (San Diego, CA). Statistical significance was determined by Student's t test for studies determining differences between RSV and RSV+EtOH alone where measured outcome was not detected in the non-infected (CTRL) or ethanol-treated (EtOH) (e.g., Fig. 1A). Two-way ANOVAs and Mann-Whitney post-tests were used to confirm differences in percent body weight change (Fig. 1A) and immune cell infiltration (Fig. 1C) among treatment groups. For all other analyses, one-way ANOVAs were used to determine differences among treatment groups and a Bonferroni post hoc analysis was used to account for multiple comparisons if the p value was <0.05. In all analyses, p values less than 0.05 were considered statistically significant. Exact p values (between 0.05 and 0.10) are reported in the text and figures to indicate statistical trends among the various treatment groups.
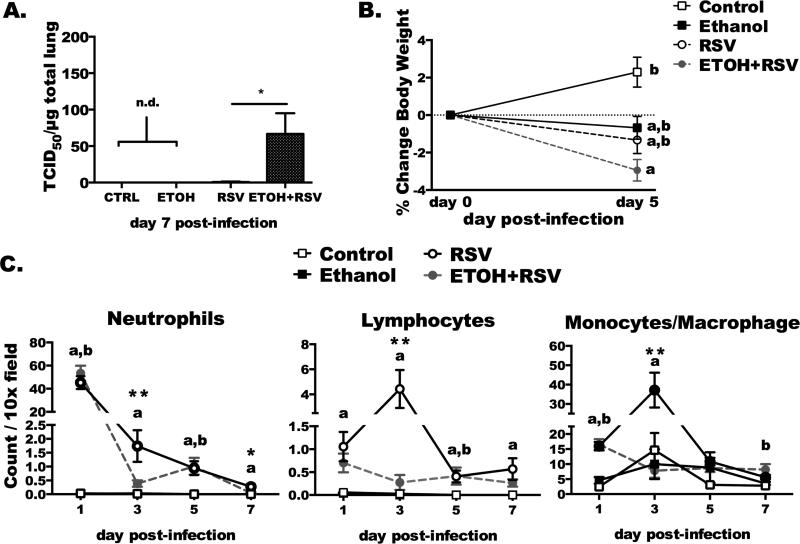
Fig. 1. Chronic ethanol consumption delays viral clearance, disrupts weight gain, and alters immune-cell kinetics to the airways following RSV infection.
Female BALB/c mice were ethanol-fed or water-fed for 6 weeks prior to RSV infection (3,250 TCID50 U/mouse). A) Lung viral titers were determined in lung homogenates (100 g/mL) at day 7 post-infection. N = 10 mice infected group, and N = 3 mice from the non-infected groups. “n.d.” denotes that virus was not detected. Statistical significance was determined by non-parametric Student's t test, where * indicates difference (p < 0.05) between EtOH+RSV treatment and RSV-infected alone. B) Percentage of body weight loss was determined over the course of the infection. a: indicates difference vs. control (p < 0.05), b: p < 0.05, indicates difference vs. EtOH+RSV-treated. Sample size equals 23–25 mice per infected group and 8–10 mice per non-infected group. C) Cell differentials determined in BAL cytospins. Counts of neutrophils, lymphocytes, or macrophage/monocytes per 10X field. Letters denote statistically significant difference between groups. a: change in body weight vs. CTRL animals. b: change in body weight compared to EtOH+RSV infected animals. Asterisks indicate a significant difference between RSV and EtOH+RSV-infected (*p < 0.05; **p < 0.01). Data are representative of 2–3 separate experiments.
Results
Chronic ethanol consumption delays viral clearance, disrupts weight gain, and alters immune cell kinetics to the airways following RSV infection
Previously, we demonstrated an increase in lung RSV titers and inflammatory mediators after chronic ethanol feeding up to day 5 post-infection (Jerrells et al., 2007). We hypothesized that recovery from RSV was delayed due to altered immune cell kinetics following RSV infection in BALB/c mice undergoing chronic ethanol feeding. Here, we demonstrate that the delay in viral clearance continues out to day 7 post-infection (Fig. 1A). At this time point, 90% (9 of 10 mice) of water-fed, RSV-infected (RSV+) mice had cleared the RSV infection from the lung; however, in ethanol-fed, RSV-infected mice (EtOH+RSV), only 10% (1 of 10 mice) had cleared the virus. As a marker of illness severity, there was a modest change in body weight following the RSV infection. Specifically, in the ethanol-fed, RSV-infected mice there was a slight decrease in body weight (RSV-infected only: −1.3% ± 0.716 vs. EtOH+RSV −2.941% ± 0.574; n = 25 and 23, respectively) following infection as compared to all other groups (Fig. 1B). RSV infection did not induce substantial body weight loss in the control-fed mice. BAL fluids were collected and cytospins were developed to quantify immune cell influx to the airways at day 1–7 after RSV infection. Significant deficiencies were determined in the EtOH+RSV mice compared to RSV at day 3 post-infection for all cell types (neutrophils, lymphocytes, and monocytes; Fig. 1C, **p < 0.01), and at day 7 significantly more neutrophils remained in the airways as well (*p < 0.05). These findings suggest that chronic ethanol feeding significantly delays viral clearance and impacts illness severity through an immune-cell mediated mechanism following RSV infection in BALB/c mice.
Airway recruitment of total CD3+ T cells, CD4+ T cells, and CD8+ T cells is altered by ethanol feeding following RSV
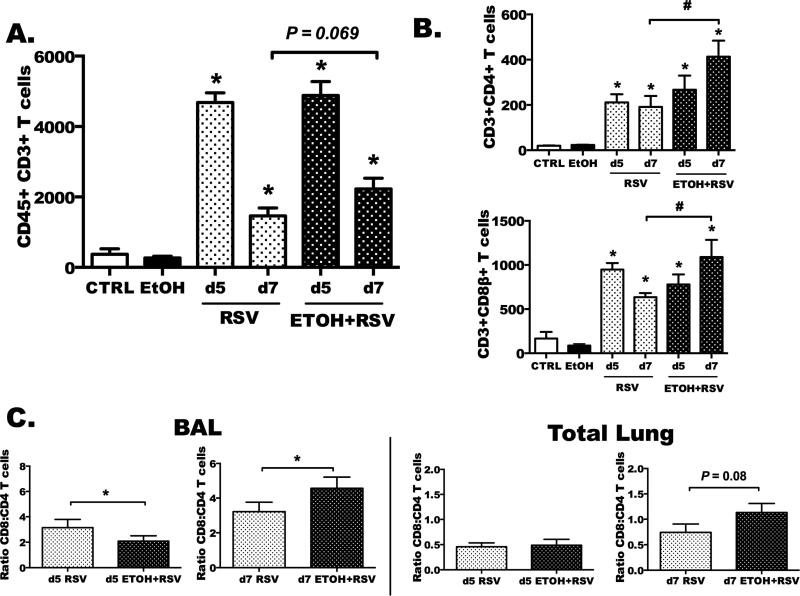
Previously, we reported a delayed influx of total lung lymphocytes at days 2 and 3 post-RSV infection following ethanol feeding (Fig. 1C) (Jerrells et al., 2007). Because of the delay in viral clearance, we hypothesized that ethanol feeding reduces the RSV-specific T-cell response mounted during infection. In these next studies, we evaluated the recruitment of total CD3+ T cells, CD3+CD4+ (helper) T cells, and CD3+CD8+ (anti-viral, cytolytic) T cells at days 5 and 7 post-infection in BAL fluids (Fig. 2). At both day 5 and day 7 after infection, we detected a significant influx of total T cells (Fig. 2A), CD4+ T cells (Fig. 2B, top) and CD8+ T cells (Fig. 2B, bottom) in the RSV-infected (RSV+) mice as compared to their water-fed (CTRL) and ethanol-fed (EtOH), non-infected controls (p < 0.05). As compared to RSV+ mice, EtOH+RSV mice demonstrated significant increases in recruitment of CD4+ and CD8+ T cells in ethanol-fed, RSV-infected mice (Fig. 2B & 2C, #p < 0.05) at day 7. There was a trend toward increased total CD3+ T-cell recruitment in ethanol-fed mice RSV-infected mice as compared to water-fed RSV-infected animals (Fig. 2A, p < 0.069). Lastly, we wanted to determine whether the ratios of CD8+ to CD4+ T cells were altered by ethanol feeding in lung lavage fluids and single cell suspensions made from non-lavaged, total lung tissue of RSV-infected mice. The ratios across the RSV-infected groups changed between day 5 and day 7 of infection such that at day 5, RSV+ mice had a higher ratio of CD8+ T cells to CD4+ T cells in BAL (Fig. 2C, left). Conversely, the EtOH+RSV mice had a greater ratio of CD8+ T cells to CD4+ T cells at day 7 in BAL (Fig. 2C, left) and total lung cell suspensions (Fig. 2C, right). Taken together with the previous data showing delayed viral clearance (Fig. 1A), these data explain the higher levels of T cells later in the course of RSV infection after ethanol feeding.
Fig. 2. Airway recruitment of total CD3+ T cells, CD4+ T cells, and CD8+ T cells is altered by ethanol feeding following RSV.
Water-fed and ethanol-fed animals were treated ± RSV and euthanized at day 5 and 7 post-infection (p.i.) to detect T-cell populations by flow cytometry in BAL and in non-lavaged total lung tissue. A) Represents CD3+ T cells and B) CD3+CD4+CD8- and CD3+CD8+CD4- T cells in BALF. C) The ratios of total CD8 to CD4 cells were determined at day 5 and day 7 in BAL (left) and non-lavaged, whole lung cell suspension (right). Results are representative of two separate experiments with n = 6–8 mice for infected groups and n = 2–3 mice per non-infected groups. Statistical significance was determined by one way ANOVA and Bonferroni post-tests. Asterisk indicates a significant RSV infection effect as compared to relative control; # represents a significant difference between RSV and EtOH+RSV (p < 0.05).
Total lung Th1 and Th2 helper cell polarization is decreased during RSV infection by ethanol feeding
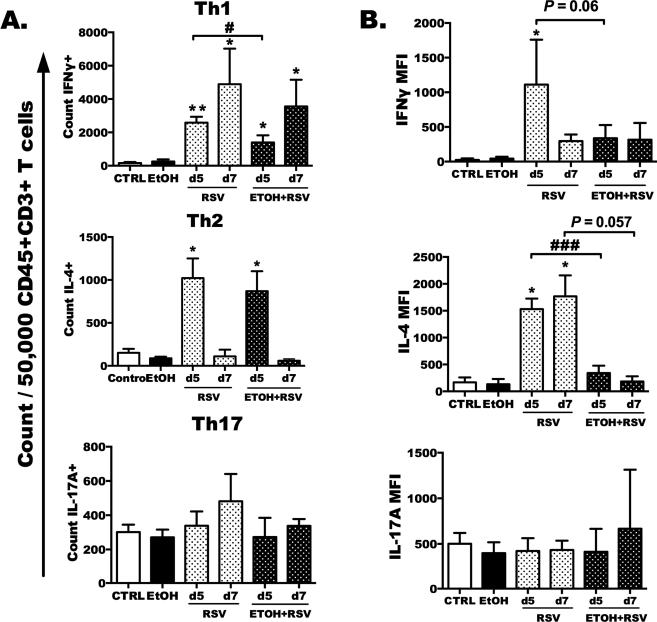
Given the altered influx of lymphocytes and T-cell populations to the airways following ethanol feeding and RSV infection at day 5 and day 7, we next hypothesized that polarization of total lung T-helper cells would decrease with ethanol feeding and RSV infection. Following ex vivo activation with PMA/Ionomycin, we demonstrated a significant increase in Th1 (CD4+IFNγ+) and Th2 (CD4+IL-4+), but not Th17 (CD4+IL-17+) cells after RSV infection as compared to relative non-infected controls (Fig. 3A, *p < 0.05 and **p < 0.01 and no change for Th17). When we compared the numbers of Th1 and Th2 cells detected per 5.0 × 104 CD45+CD3+ T cells, we detected fewer Th1 cells (#p < 0.05) in the lungs of EtOH+RSV mice as compared to RSV+ mice from day 5 (no difference at day 7).
Fig. 3. Total lung Th1 and Th2 helper cell polarization is decreased during RSV infection by ethanol feeding.
Ethanol-fed or water-fed RSV-infected mice were euthanized on days 5 and 7, and lung T cells were isolated, stimulated with PMA/Ionomycin, and stained for intracellular IFNγ, IL-4, and IL-17A. A) Bar graphs depict mean ± SEM of the count of CD45+CD3+CD4+ T cells that were positive for IFNγ (Th1, top) and IL-4 (Th2, middle) and IL-17A (bottom) per 50,000 CD45+CD3+CD4+ events. B) Bar graph depicts the cytokine median fluorescence intensity (MFI) per cell basis minus the media control. Results are representative of two independent experiments with a total of 6–10 mice per treatment group. Statistical analysis was carried out using a one-way ANOVA followed by Bonferroni post-test. Asterisks are indicative of a significant infection effect as compared to relative control (*p < 0.05 or **p < 0.01), and # or ### indicates a difference between RSV and EtOH+RSV (p < 0.05 or 0.001).
We also evaluated the exact level of intracellular cytokine production by each of the T-helper subsets on a per cell basis (reported as mean fluorescence intensity, or MFI) at both day 5 and day 7. The type 1 helper T-cell response, or IFNγ MFI, was significantly reduced between the RSV+ and EtOH+RSV mice (Fig. 3B, top, #p < 0.05) at day 5 of RSV infection. IL-4 production by CD4+IL-4+ (Th2) cells was significantly decreased at day 5 post-infection with ethanol feeding (Fig. 3B, middle, ###p < 0.001). The Th17 response, as measured by IL-17A production by CD4+IL-17A+ cells, was not different among the groups (Fig. 3B, bottom). T-helper cell responses were measured again at day 7 in lung-isolated T cells, but only IL-4 MFI of Th2 cells remained decreased (Fig. 3B, middle). All other T-helper populations and cytokine production were comparable among the RSV-infected groups at day 7 after infection.
RSV-specific activation of lung CD8+ T cells, and IFNγ production in the lung, are attenuated by chronic ethanol feeding
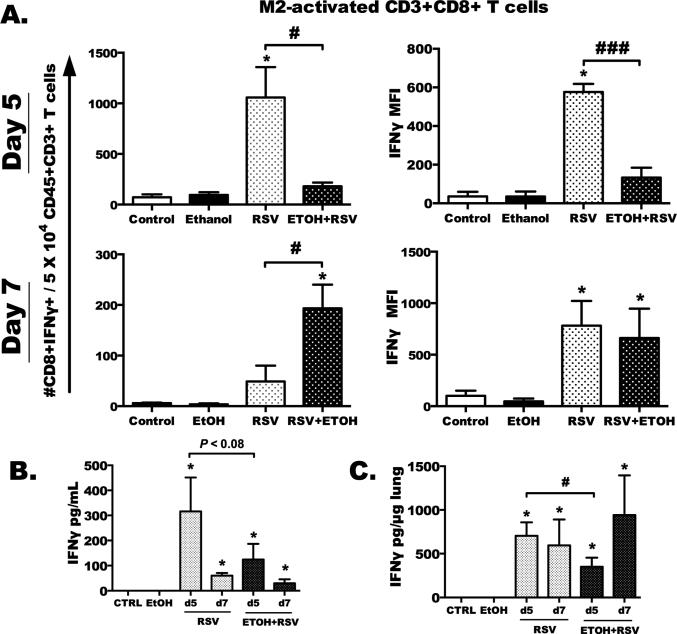
In these studies, we sought to determine the magnitude of the RSV-specific CD8 T-cell response using the dominant RSV peptide (M282–90) to stimulate CD8 T cells ex vivo. We hypothesized that this response would be decreased with ethanol feeding and RSV infection. CD8+IFNγ+ cells were determined by flow cytometry, and the numbers of M2-activated cells detected in the lungs were increased with RSV infection in water-fed animals only at day 5 post-infection (Fig. 4A, top and left, *p < 0.05), and not at day 7 (Fig. 4A, bottom and left). A significant decrease in the number of CD8+IFNγ+ cells was detected with ethanol-fed, RSV-infected mice (#p < 0.05) at day 5, and ethanol-fed, RSV-infected mice demonstrated a significant decrease in IFNγ expression as compared to RSV-infected alone at day 5 (Fig. 4A, top and right, ###p < 0.001). IFNγ production in the lungs and BAL was also reduced at day 5 post-infection in ethanol-fed, RSV-infected mice (Fig. 4B, 4C, #p < 0.05 [total lung] and p < 0.08 [BAL]). At day 7 after RSV infection, while we detected a significant number of M2-specific CD8+IFNγ+ T cells in the lungs of ethanol-treated, RSV-infected mice (Fig. 4A, bottom, #p < 0.05), we detected no change in IFNγ MFI in the RSV vs. EtOH+RSV CD8+IFNγ+ cells (Fig. 4A, bottom and right). In addition, there was no difference in IFNγ lung protein (BAL or lung tissue) levels between water-fed and ethanol-fed RSV-infected animals at day 7 (Fig. 4B, 4C). Given the virus remaining in lungs at this time point, however, increases in M2-specific CD8+IFNγ+ cell numbers are not surprising for the ethanol-treated, RSV-infected mice compared to water-fed RSV-infected mice. Taken together, with the delayed viral clearance and decreased T-helper response, this suggests that specific RSV immunity, mediated through the IFNγ pathway, may be defective in chronic alcohol-fed mice.
Fig. 4. RSV-specific activation of lung CD8+ T cells and IFNγ is attenuated by chronic ethanol feeding.
Total lung CD3+ T cells were isolated from water-fed and ethanol-fed +/− RSV-infected animals at day 5 and day 7, then stimulated with the RSV-specific, immunodominant M2 peptide (1 M) for 6 h. A) Counts of CD8+IFNγ+ T cells per 50,000 total CD45+CD3+CD8+ T cells (left), and corresponding intracellular IFNγ MFI (right) for day 5 (top graphs) and day 7 (bottom graphs). B) Total IFNγ protein in BAL and C) total lung homogenate determined by ELISA. Statistical significance was determined using a one-way ANOVA and Bonferroni post-test. Asterisk indicates a significant infection effect compared to relative non-infected control (*p < 0.05). # or ### indicates a significant difference (p < 0.05 and 0.001) between infected groups by Bonferroni post-test. Results are representative of two separate experiments, with 6–10 mice per group in each experiment.
Discussion
Chronic ethanol consumption has been recognized as a comorbidity for respiratory illness for centuries. Respiratory syncytial virus is an important viral pathogen not only in children, but also in immune-compromised patients, the elderly, and young adults (Falsey, 2005; Falsey et al., 2006; Falsey, Hennessey, Formica, Cox, & Walsh, 2005; Falsey & Walsh, 2000, 2005; Kulkarni et al., 2016; O'Shea, Pipkin, Cane, & Gray, 2007; O'Shea, Ryan, Hawksworth, Alsip, & Gray, 2005). Here we showed altered immune responses to RSV in BALB/c mice that have consumed 18% ethanol in their drinking water for 6 weeks. Lymphocytic recruitment to the airways was delayed with ethanol feeding, and virus persisted in the lung out to day 7 post-infection in ethanol-fed mice (Fig. 1). Ethanol feeding decreased the Th1 response commonly described for all viral infections (Fig. 3). Similarly, ethanol feeding blunted the Th2 response, which is characteristic only to RSV infections (Fig. 3). Finally, we examined the response of CD8+ T cells to the immunodominant RSV peptide, M2, and discovered a significant decrease in the IFNγ production by these cells as well as a significant reduction in total lung IFNγ production (Fig. 4). Because this cell population is necessary for cytolytic killing of RSV-infected cells, and IFNγ is pertinent for activating other immune-mediated events (macrophage phagocytosis, B-cell class switching), this reduced response explains the delay in viral clearance and prolonged illness in the mice.
General lymphocytic recruitment was altered by ethanol treatment in our previous published data at time points as early as 48 h (Jerrells et al., 2007). Others report decreased IFNγ production by influenza-activated lung CD8+ T cells isolated from ethanol-fed influenza-infected mice (Meyerholz et al., 2008). These type 1 responses are necessary for fighting bacterial pathogens (e.g. M. tuberculosis and K. pneumoniae) as well (Greenberger et al., 1996; Moore, Perry, Getsoian, Newstead, & Standiford, 2002). This coincides well with the findings of our present work where we detected reduced IFNγ (Fig. 4). In addition, alterations to cell kinetics have been reported for neutrophils in alcohol-feeding models that do not involve viral pathogens (Raasch et al., 2010; Siggins et al., 2011). Thus, the delayed cellular influx into the lungs and to airways is not surprising. Much work has focused on understanding the basic changes ethanol exposure has on the airway epithelium; ciliary beating and clearance responses are decreased after prolong exposure to alcohol, and IL-8 (neutrophil chemoattractant) release is decreased in the lungs as well (Allen-Gipson et al., 2004). Here we expand upon those findings and show that early kinetics of lung immune-cell infiltration and later T-cell recruitment is delayed by ethanol feeding after RSV infection (Fig. 1 & Fig. 2). It is well reported that BALB/c mice can clear RSV infection within 5 days (Lotz & Peebles, 2012), yet at day 7 after RSV infection, our ethanol-fed mice are still actively infected with live virus (Fig. 1). There may be a combination of alcohol-related defects increasing susceptibility to RSV infection. However, defining the various alcohol-related defects in concert is a current limitation of these studies.
The necessity for early innate immune response in shaping the adaptive/specific immune response, and even long-term immune memory, weeks after infection has been confirmed (Schoenberger, Pulendran, & Katsikis, 2012; Smit et al., 2008; Smit, Rudd, & Lukacs, 2006). Thus, the defect in the downstream anti-viral CD8 T cells and viral clearance may be the result of the late recruitment of innate immune cells (e.g., dendritic cells, inflammatory monocytes), which serve important functions in activating the specific immune response. As suggested by our data and data from the ethanol-feeding influenza viral infection model (Meyerholz et al., 2008), viral processing and presentation by the antigen-presenting cell (APC) populations may be the specific cause for the decreased adaptive immune response. Further studies determining APC recruitment and T-cell activation by these cells in secondary immune organs of ethanolfed animals may have merit. However it is unknown whether the results in the present study are the result of an intrinsic T-cell defect or an environmental effect. It is likely a combination of both, yet it is plausible that transferred T cells can restore the anti-viral protection in the lungs. To our knowledge, no one has attempted to rescue the T-cell phenotype by transferring memory or activated T cells from naïve or previously RSV-challenged hosts. These studies are tedious, but may prove therapeutically important for individuals who succumb quickly to respiratory viral infections.
Notably, we detected a blunted IL-4 production by Th2 cells (Fig. 3). Perhaps the decrease in IL-4 may indicate reduced regulation of inflammation, as IL-4 responses can have an anti-inflammatory effect, especially if IL-10 is detected as well. Recently, Weiss et al. (2011) described a protective role for IL-10-producing CD4-helper T cells in lungs of RSV-infected mice (Weiss, Christiaansen, Fulton, Meyerholz, & Varga, 2011). This IL-10-producing population was composed of a subset of CD4+ cells that expressed FOXP3+ (a marker of T regulatory cells), and a CD4+FOXP3- population. In IL-10 knockout studies, the burden of RSV infection in the lungs was increased in the absence of IL-10. This may explain our recent finding of a modest increase in IFNγ-producing CD8+ cells detected in the lungs at day 7 post-infection, yet a reduced level of IL-10 in BAL and lungs at the same time point (data not shown). The presence of IFNγ indicates an ensuing chronic RSV infection, considering the amount of virus still detected in the lungs of the ethanol-fed mice. We anticipate that the IL-10 response may be delayed as well and therefore not present to limit the inflammatory state in the lungs later in infection. Further studies are needed to delineate the loss of IL-4 in conjunction with IL-10 and examine the contribution of T regulatory cells in the lungs of the ethanol-fed, RSV-infected mice.
In summary, this work adds to the previously recognized finding of the comorbidity of alcohol on the lungs and immune system during respiratory infections. Herein, we have intricately defined a delayed viral clearance of RSV and shown extensively that T-helper cell polarization and the anti-viral CD8 responses are delayed, yet not completely defective in alcohol-treated animals. Chronic alcohol abusers are often hospitalized with common respiratory infections, which necessitate a functioning T-cell repertoire. Therefore, future studies are planned to expand upon this work to potentially identify therapeutic interventions for these individuals.
Highlights.
Immune-cell kinetics into the lungs are delayed in BALB/c mice with ethanol consumption following RSV infection.
We show that T-cell recruitment to the airways is reduced.
T-helper cell polarization and RSV-specific CD8+ T-cell activation are both reduced due to chronic alcohol consumption.
Acknowledgments
Funding sources for this research are as follows: NIAAA AA017993 R01 (T.A.W.), VA I01 BX000728 (T.A.W.), NIAAA R01 AA008769 (J.H.S.), F32 FAA019859 NIAAA (S.M.S.) and NIEHS R01 ES019325 (J.A.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen-Gipson DS, Romberger DJ, Forget MA, May KL, Sisson JH, Wyatt TA. IL-8 inhibits isoproterenol-stimulated ciliary beat frequency in bovine bronchial epithelial cells. Journal of Aerosol Medicine. 2004;17:107–115. doi: 10.1089/0894268041457138. [DOI] [PubMed] [Google Scholar]
- Al-Sanouri I, Dikin M, Soubani AO. Critical care aspects of alcohol abuse. Southern Medical Journal. 2005;98:372–381. doi: 10.1097/01.SMJ.0000154769.33508.20. [DOI] [PubMed] [Google Scholar]
- Berger JP, Simet SM, DeVasure JM, Boten JA, Sweeter JM, Kharbanda KK, et al. Malondialdehyde-acetaldehyde (MAA) adducted proteins bind to scavenger receptor A in airway epithelial cells. Alcohol. 2014;48:493–500. doi: 10.1016/j.alcohol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. The Journal of Experimental Medicine. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Alcohol-attributable deaths and years of potential life lost -- United States, 2001. Morbidity and Mortality Weekly Report. 2004;53:866–870. [PubMed] [Google Scholar]
- Christiaansen AF, Knudson CJ, Weiss KA, Varga SM. The CD4 T cell response to respiratory syncytial virus infection. Immunologic Research. 2014;59:109–117. doi: 10.1007/s12026-014-8540-1. [DOI] [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, et al. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcoholism: Clinical and Experimental Research. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT, Zhu X, Coleman RA, Ballas ZK, Waldschmidt TJ, Ray NB, et al. T-cell activation after chronic ethanol ingestion in mice. Alcohol. 2004;33:175–181. doi: 10.1016/j.alcohol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunological Reviews. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. American Journal of Respiratory Cell and Molecular Biology. 2007;36:452–459. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR. Respiratory syncytial virus infection in elderly and high-risk adults. Experimental Lung Research. 2005;31(Suppl 1):77. [PubMed] [Google Scholar]
- Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2006;173:639–643. doi: 10.1164/rccm.200510-1681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. The New England Journal of Medicine. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clinical Microbiology Reviews. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in elderly adults. Drugs & Aging. 2005;22:577–587. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble L, Mason CM, Nelson S. The effects of alcohol on immunity and bacterial infection in the lung. Médecine et Maladies Infectieuses. 2006;36:72–77. doi: 10.1016/j.medmal.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and Alcohol Dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:361–368. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- Greenberger MJ, Kunkel SL, Strieter RM, Lukacs NW, Bramson J, Gauldie J, et al. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. Journal of Immunology. 1996;157:3006–3012. [PubMed] [Google Scholar]
- Hashimoto K, Mori S, Hashimoto Y, Kaneko H, Ishibashi K, Ishioka K, et al. DSCG reduces RSV-induced illness in RSV-infected mice. Journal of Medical Virology. 2009;81:354–361. doi: 10.1002/jmv.21378. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Pavlik JA, DeVasure J, Vidlak D, Costello A, Strachota JM, et al. Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory syncytial virus in mice. Alcohol. 2007;41:357–369. doi: 10.1016/j.alcohol.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni H, Smith CM, Lee Ddo H, Hirst RA, Easton AJ, O'Callaghan C. Evidence of Respiratory Syncytial Virus Spread by Aerosol. Time to Revisit Infection Control Strategies? American Journal of Respiratory and Critical Care Medicine. 2016;194:308–316. doi: 10.1164/rccm.201509-1833OC. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Metabolism of ethanol and its effects upon the liver. Quaderni Sclavo di Diagnostica Clinica e di Laboratorio. 1971;7:861–896. [PubMed] [Google Scholar]
- Lotz MT, Peebles RS., Jr. Mechanisms of respiratory syncytial virus modulation of airway immune responses. Current Allergy and Asthma Reports. 2012;12:380–387. doi: 10.1007/s11882-012-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. Journal of Immunology. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TA, Perry ML, Getsoian AG, Newstead MW, Standiford TJ. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infection and Immunity. 2002;70:6310–6318. doi: 10.1128/IAI.70.11.6310-6318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz JL, McCarthy CA, Clark ME, Hall CB. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cells. Journal of Virology. 1991;65:4494–4497. doi: 10.1128/jvi.65.8.4494-4497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. 10th Special Report to the U.S. Congress on Alcohol and Health. National Institute on Alcohol Abuse and Alcoholism; 2000. [Google Scholar]
- Oldenburg PJ, Poole JA, Sisson JH. Alcohol reduces airway hyperresponsiveness (AHR) and allergic airway inflammation in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2012;302:L308–315. doi: 10.1152/ajplung.00077.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea MK, Pipkin C, Cane PA, Gray GC. Respiratory syncytial virus: an important cause of acute respiratory illness among young adults undergoing military training. Influenza and Other Respiratory Viruses. 2007;1:193–197. doi: 10.1111/j.1750-2659.2007.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea MK, Ryan MA, Hawksworth AW, Alsip BJ, Gray GC. Symptomatic respiratory syncytial virus infection in previously healthy young adults living in a crowded military environment. Clinical Infectious Diseases. 2005;41:311–317. doi: 10.1086/431591. [DOI] [PubMed] [Google Scholar]
- Peebles RS, Jr., Moore ML. A mechanistic advance in understanding RSV pathogenesis, but still a long way from therapy. American Journal of Respiratory Cell and Molecular Biology. 2007;37:375–377. doi: 10.1165/rcmb.2007-0003ED. [DOI] [PubMed] [Google Scholar]
- Poole JA, Gleason AM, Bauer C, West WW, Alexis N, Reynolds SJ, et al. αβ T cells and a mixed Th1/Th17 response are important in organic dust-induced airway disease. Annals of Allergy, Asthma & Immunology. 2012;109:266–273. doi: 10.1016/j.anai.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasch CE, Zhang P, Siggins RW, 2nd, LaMotte LR, Nelson S, Bagby GJ. Acute alcohol intoxication impairs the hematopoietic precursor cell response to pneumococcal pneumonia. Alcoholism: Clinical and Experimental Research. 2010;34:2035–2043. doi: 10.1111/j.1530-0277.2010.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E, Lieber CS. Ethanol and the liver: an example of injury and adaptation. Human Pathology. 1971;2:343–344. doi: 10.1016/s0046-8177(71)80001-1. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Pulendran B, Katsikis PD. 4th Aegean Conference on the Crossroads between Innate and Adaptive Immunity. Nature Immunology. 2012;13:7–10. doi: 10.1038/ni.2192. [DOI] [PubMed] [Google Scholar]
- Shellito JE. Alcohol and host defense against pulmonary infection with Pneumocystis carinii. Alcoholism: Clinical and Experimental Research. 1998;22:208S–211. doi: 10.1111/j.1530-0277.1998.tb04003.x. [DOI] [PubMed] [Google Scholar]
- Shellito JE, Olariu R. Alcohol decreases T-lymphocyte migration into lung tissue in response to Pneumocystis carinii and depletes T-lymphocyte numbers in the spleens of mice. Alcoholism: Clinical and Experimental Research. 1998;22:658–663. doi: 10.1111/j.1530-0277.1998.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Siggins RW, Melvan JN, Welsh DA, Bagby GJ, Nelson S, Zhang P. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. Journal of Immunology. 2011;186:4306–4313. doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simet SM, Sisson JH. Alcohol's Effects on Lung Health and Immunity. Alcohol Research: Current Reviews. 2015;37:199–208. [PMC free article] [PubMed] [Google Scholar]
- Simet SM, Wyatt TA, DeVasure J, Yanov D, Allen-Gipson D, Sisson JH. Alcohol increases the permeability of airway epithelial tight junctions in Beas-2B and NHBE cells. Alcoholism: Clinical and Experimental Research. 2012;36:432–442. doi: 10.1111/j.1530-0277.2011.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrett SJ, Niederman MS, Fein AM. Respiratory infections and acute lung injury in systemic illness. Clinics in Chest Medicine. 1989;10:469–502. [PubMed] [Google Scholar]
- Smit JJ, Lindell DM, Boon L, Kool M, Lambrecht BN, Lukacs NW. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PloS One. 2008;3:e1720. doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. The Journal of Experimental Medicine. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. Journal of Immunology. 1997;159:5197–5200. [PubMed] [Google Scholar]
- Vander Top EA, Wyatt TA, Gentry-Nielsen MJ. Smoke exposure exacerbates an ethanol-induced defect in mucociliary clearance of Streptococcus pneumoniae. Alcoholism: Clinical and Experimental Research. 2005;29:882–887. doi: 10.1097/01.alc.0000164364.35682.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga SM, Braciale TJ. RSV-induced immunopathology: dynamic interplay between the virus and host immune response. Virology. 2002;295:203–207. doi: 10.1006/viro.2002.1382. [DOI] [PubMed] [Google Scholar]
- Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15:637–646. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- Warren KJ, Olson MM, Thompson NJ, Cahill ML, Wyatt TA, Yoon KJ, et al. Exercise Improves Host Response to Influenza Viral Infection in Obese and Non-Obese Mice through Different Mechanisms. PloS One. 2015;10:e0129713. doi: 10.1371/journal.pone.0129713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, Varga SM. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. Journal of Immunology. 2011;187:3145–3154. doi: 10.4049/jimmunol.1100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Pavlik J, Fox L, Scarbrough C, Sale WS, Sisson JH, et al. Alcohol-induced ciliary dysfunction targets the outer dynein arm. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2015;308:L569–576. doi: 10.1152/ajplung.00257.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Roberts AD, Woodland DL. Antibody-independent antiviral function of memory CD4+ T cells in vivo requires regulatory signals from CD8+ effector T cells. Journal of Immunology. 2001;167:1379–1386. doi: 10.4049/jimmunol.167.3.1379. [DOI] [PubMed] [Google Scholar]