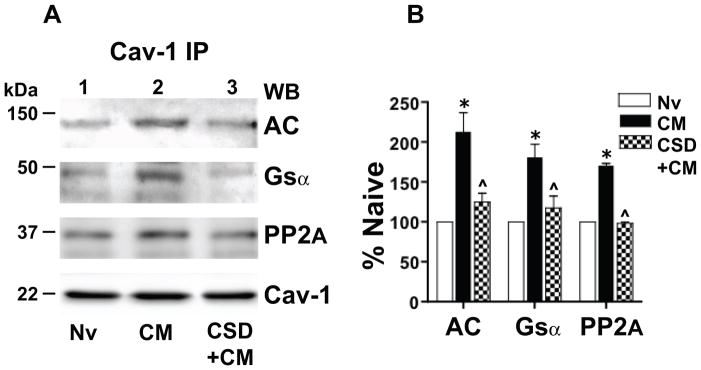
Figure 7.
Chronic morphine augments association of Gsα, protein phosphatase 2A (PP2A) and adenylyl cyclase (AC) with Cav-1 in rat spinal cord. Their association is substantially attenuated/eliminated by blocking the Cav-1 scaffolding domain. A: The Triton insoluble fraction obtained was obtained from lumbar spinal cord membranes of opioid naïve rats (Nv, Lane 1), i.t. chronic morphine (CM)-treated rats (lane 2), or rats treated chronically with i.t. morphine concomitant with 10 μg of the Cav-1 scaffolding domain peptide CSD (CSD+CM) (lane 3; see Methods). The Triton insoluble fraction was solubilized and subjected immunoprecipitation using anti-Cav-1 antibodies. Following SDS PAGE and electrotransfer of proteins, regions of the nitrocellulose membrane corresponding to AC, Gsα, PP2A and Cav-1 were cut out and Western blotted with their corresponding antibodies. Quantification of band intensity was normalized to the amount of their corresponding directly IPed Cav-1 protein. B: The content of Gsα, PP2A and AC in the Cav-1 IP obtained from spinal cord of the above treated rats is expressed as a percent of their content in Cav-1 IP obtained from spinal cord of opioid naïve rats. A regimen of chronic in vivo morphine that produces spinal analgesic tolerance increased the association of Gsα, PP2A and AC with Cav-1 within caveolae/Cav-1 scaffolds of rat spinal cord. This increment was essentially eliminated by concomitant treatment with CSD. n=3; *=p<0.001 for CM vs. Nv; ^= p<0.01 for CM+CSD vs. CM.