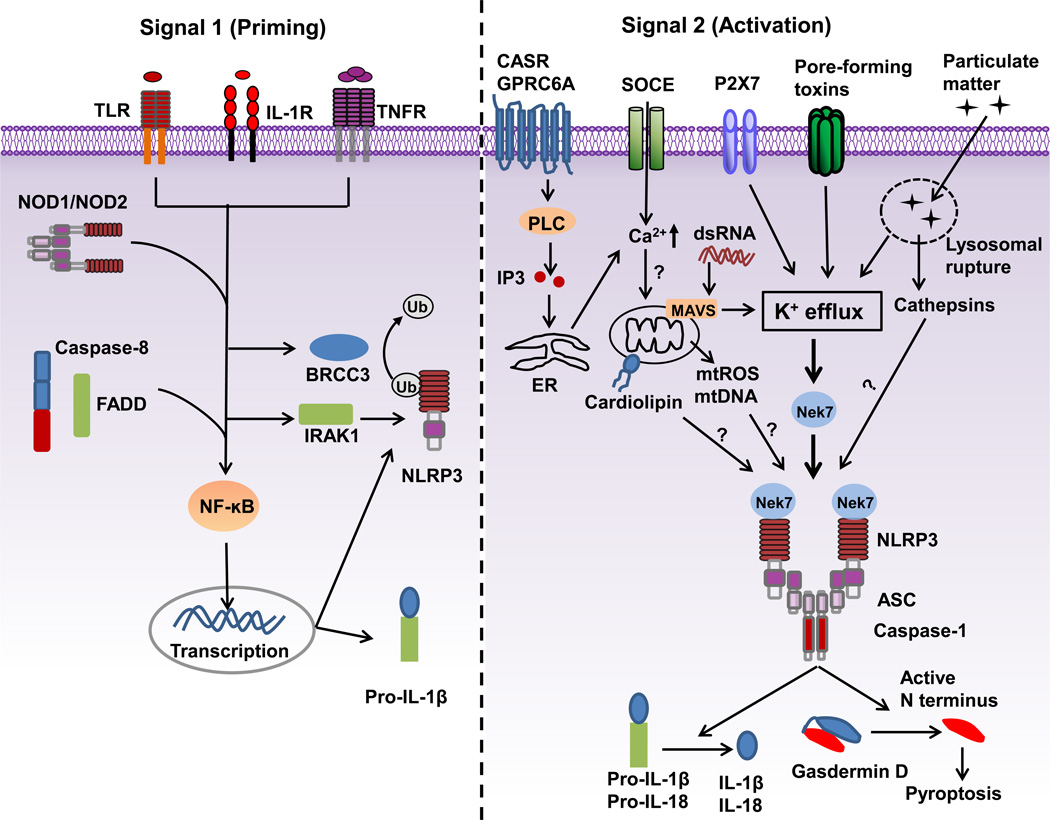
Figure 1.
A two-signal model for NLRP3 inflammasome activation. Signal 1 (priming, left) is provided by microbial molecules or endogenous cytokines and leads to the upregulation of NLRP3 and pro-IL-1β through the activation of transcriptional factor NF-κB. Caspase-8 and FADD are involved in priming via the regulation of the NF-κB activation pathway. BRCC3 (a deubiquitinase) and IRAK1 regulate NLRP3 inflammasome activation independently of transcription. Signal 2 (activation, right) is provided by a plethora of stimuli, such as ATP, pore-forming toxins, viral RNA and particulate matter, and activates the NLRP3 inflammasome. A number of signaling events have been proposed as the key mechanism of NLRP3 activation. Most NLRP3 stimuli induce K+ efflux that is necessary and sufficient for NLRP3 activation. Ca2+ signaling is proposed to cause mitochondrial dysfunction and is implicated in NLRP3 inflammasome activation. Mitochondrial dysfunction-derived signals, such as reactive oxygen species (mtROS), oxidized mtDNA or externalization of phospholipid cardiolipin have also been suggested to mediate NLRP3 activation. Mitochondrial adaptor MAVS mediates NLRP3 activation induced by RNA viruses. Particulate matter activates NLRP3 through lysosomal rupture-induced K+ efflux and perhaps the release of cathepsins. Nek7 is an essential regulator of the NLRP3 inflammasome. TLR: Toll-like receptor; IL-1R: receptor for IL-1β; TNFR: receptor for tumor-necrosis factor; BRCC3: Lys-63-specific deubiquitinase BRCC36; FADD: FAS-associated death domain protein; IRAK1:Interleukin-1 receptor-associated kinase 1.