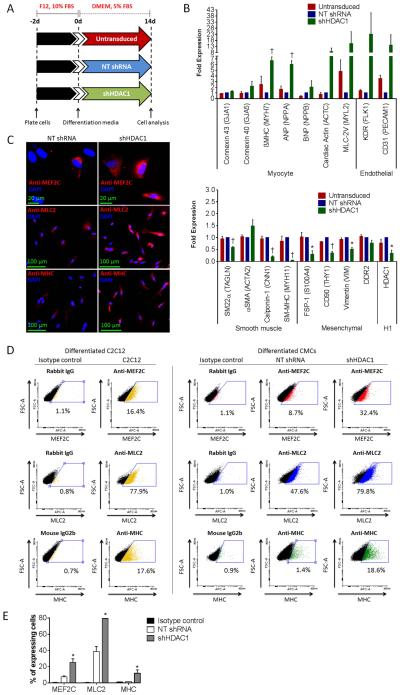
Figure 6.
HDAC1-depleted CMCs exhibit heightened commitment towards a myocyte- and endothelial-cell-like fate following their differentiation in vitro. (A): In vitro cardiogenic differentiation schematic and timeline. Untransduced, NT shRNA control, or shHDAC1 transduced CMCs were cultured under reduced serum conditions for 14 d to induce cardiogenic differentiation. (B): Real-time qPCR assays assessing the expression of cardiovascular cell lineage markers (myocyte, endothelial, smooth muscle and mesenchymal/fibroblast) in differentiated CMCs. Values are mean (n=3) ± SEM. * P <0.05 (Relative to NT shRNA) and † P <0.005 (Relative to NT shRNA). (C): Immunocytochemical (n=3) and (D): flow cytometric (n=3) detection of myocyte lineage-specific proteins [Mef2C (myocyte-specific enhancer factor 2C), MLC2 (cardiac myosin light chain 2), and MHC (cardiac myosin heavy chain)] in differentiated CMCs. Differentiated C2C12 myoblasts (cultured under reduced serum conditions for 14 d) served as a positive control in FACS. (E): Graph depicting flow cytometric-based quantification of the percentage of differentiated CMCs expressing Mef2C, MLC2, or MHC proteins. Values are mean (n=3) ± SEM. * P <0.05 (shHDAC1 vs. NT shRNA).