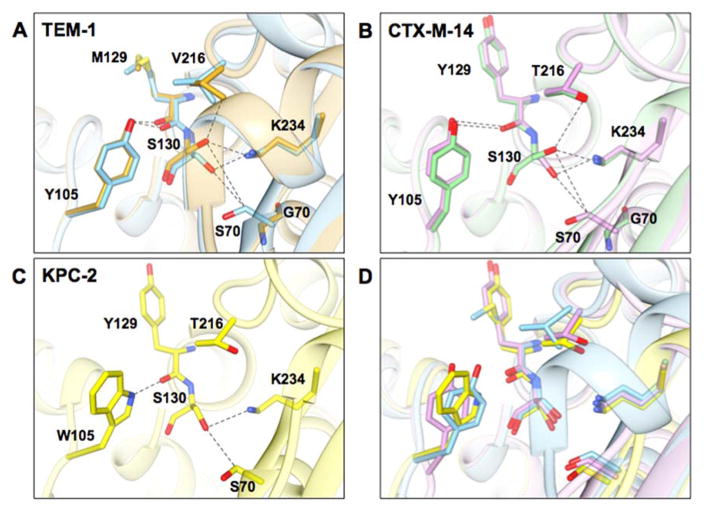
Figure 6.
Structural representations of the active sites of class A β-lactamases from this study. A) Wild-type TEM-1 (PDB ID: 1ZG4) is shown in blue and TEM-1 S70G in gold (PDB ID: 1ZG6). B) Wild-type CTX-M-14 (PDB ID: 1YLT) is shown in purple and the S70G mutant (PDB ID: 4PM6) in light green. C) Wild type KPC-2 (PDB ID: 2OV5) is shown in yellow. D) Structural alignment of TEM-1, CTX-M-14, and KPC-2 β-lactamases. Selected residues are represented in stick and labeled. Dashed lines represent distances between atoms from 2.8 to 3.8 angstroms. Note that KPC-2 has a tryptophan at position 105 while CTX-M-14 and TEM-1 have tyrosine at this position. In addition, CTX-M-14 and KPC-2 have tyrosine and threonine at positions 129 and 216 while TEM-1 has methionine and valine at these positions.