Abstract
Background: Surgical debridement and broad-spectrum empiric antibiotics are first-line therapy for necrotizing soft tissue infections (NSTI). The objective of this multi-center retrospective review was to evaluate antimicrobial agent initiation and duration and compare outcomes in the treatment of patients with NSTI.
Patients and Methods: This review included adults with NSTI, as indicated by International Classification of Diseases, 9th Edition, Clinical Modification codes 728.86, 608.33, or 040.0, who were admitted to three academic institutions between 1/1/09 and 5/15/14. Demographics, antibiotic practices, operative management, and clinical outcomes were compared.
Results: A total of 341 patients were identified at the three centers. Subjects were comparable in age (median 53 years, p = 0.14), gender (67% male, p = 0.57) and body mass index (median 31.9 (p = 0.31) between sites. No significant difference was found in time from admission to start of empiric antibiotic therapy between the three centers (median 1 d for each, p = 0.70), but duration of antibiotic therapy was significantly different (Site A = 16 d, Site B = 12 d, Site C = 9 d, medians, p < 0.001). Although total number of operations differed between sites (median of two at Sites A and B, three at Site C, p = 0.001), sites consistently operated on the day of patient arrival to their facility, and the number of debridements did not differ (median of two for all sites, p = 0.10). Mortality rate (Site A = 22%, Site B = 18%, and Site C = 9%, p = 0.02) and length of stay for survivors (Site A = 29 d, Site B = 16 d, Site C = 19 d, medians, p = 0.001) was significantly different among centers.
Conclusions: Variation in antibiotic duration between centers with expertise in the care of NSTI illustrates how little is known about best care practices for patients with NSTI. Future studies should emphasize development of evidence-based practices for NSTI management to further improve the outcomes of this complex group of patients.
Keywords: : antibiotic therapy, necrotizing soft tissue infection, necrotizing fasciitis, outcomes
Necrotizing soft tissue infections (NSTI) are a group of uncommon fulminant infections that require prompt surgical debridement as well as systemic support to mitigate the high rates of morbidity and mortality [1–3]. Infectious Diseases Society of America (IDSA) guidelines support early and aggressive surgical excision of infected tissue accompanied by appropriate antibiotic therapy [4]. Recommendations for antibiotic duration include treatment until the time of complete surgical debridement, clinical resolution, and absence of fever for 48–72 hours [4]; actual practice patterns are largely unknown and clinical trials of antibiotic management in NSTI are lacking.
Chastre et al. [5] showed comparable outcomes in patients with ventilator-associated pneumonia between 8- and 15-day antibiotic regimens; however, similar comparisons of drug therapy are lacking in NSTI. Most NSTI studies have focused instead on identification of this relatively rare disease [3,6,7] or prediction of death [8,9]. Because of the relative paucity of NSTI cases, larger series involve multiple centers experienced in the treatment of critically ill patients with complex surgical infection.
Previous multi-center studies have focused on creating a predictive mortality model [8,9] or on evaluating a single treatment modality [10,11]. Kao et al. [6] broadly described presentation, treatment, and outcome from NSTI at six academic hospitals in Texas. They found that patient characteristics, particularly age and severity of disease, were independent predictors of death and postulated that practice variations may affect outcome. The objective of our study was to perform a review of practices in the clinical management of NSTI with a focus on antibiotic duration and timing and operative management among three academic centers with associated regional burn centers in geographically disparate regions.
Patients and Methods
NSTI identification
A retrospective review of patients who were hospitalized with NSTI at three academic institutions with American Burn Association/American College of Surgeons verified burn centers was performed (Site A, B, C). These three centers were selected for their relatively high volume of NSTI cases encountered and diverse geographic locations. After Institutional Review Board approval, we identified adult patients (age >18 y) admitted between January 1, 2009 and May 15, 2014 with an International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9 CM) code for (040.0) gas gangrene, (608.83) Fournier gangrene, or (728.86) necrotizing fasciitis. Patient charts were manually reviewed at each location to confirm that the patient's primary reason for admission was NSTI.
Data collection and variables
Study data were collected and managed using REDCap (Research Electronic Data Capture), an electronic data capture tool, which is a secure, web-based application designed to support data capture for research studies It provides (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources [12].
Variables collected include basic demographics, comorbidities, clinical course, and outcomes. Missing data on comorbidities at the time of admission were assumed to be normal. Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were based on parameters abstracted from the first 24 hours of admission.
Antibiotic drug days were counted as each day that a patient received one or more antibiotics; administration of multiple antibiotics on the same day counted as a single antibiotic day. Identification of an operation as a soft tissue debridement required that this was the primary procedure listed in the operative note.
Our primary objective was to evaluate the relation between antibiotic use patterns and death in NSTIs. Secondary outcomes included number of post-operative complications, number of nosocomial infections, and length of stay (LOS). Post-operative complications were defined as complications consequent to the patient's operation. Nosocomial infections such as pneumonia and urinary tract infection (UTI) required positive cultures and a treatment intervention.
Statistical analysis
Values were reported as a median with interquartile range (IQR) or percentage unless otherwise stated. Chi-square or Fisher exact was used for categorical or nominal variables. For continuous variables, the Kruskal-Wallis test was used when comparing sites (three comparators) versus comparing survivors versus non-survivors (two comparators) Rank-sum test was used. To estimate survival time, the Kaplan-Meier method and risk table were included. Patients who had a LOS greater than 90 days (n = 5) were excluded from the Kaplan-Meier figure to prevent a misleading estimate with respect to sample size. We used STATA 13.0 (College Station, TX) for all analysis and considered a two-sided alpha of 0.05 as the threshold for statistical significance.
Results
Demographic and clinical characteristics
A total of 341 patients with NSTI were admitted to Site A (n = 60), Site B (n = 116), and Site C (n = 165) during the review period. Patients at the three centers were comparable in age, gender, and body mass index (Table 1). The vast majority of patients (92%) had medical comorbidities. The three most common comorbidities were diabetic mellitus (50%), hypertension necessitating medication (48%), and current smoker (33%).
Table 1.
Demographics and Severity of Illness for All Sites*
| Variable | p | Total n = 341 | Site A n = 60 (18%) | Site B n = 116 (34%) | Site C n = 165 (48%) |
|---|---|---|---|---|---|
| Male | 0.57 | 229 (67%) | 43 (72%) | 74 (64%) | 112 (68%) |
| Age | 0.14 | 53 (44–60) | 55 (49–59) | 51 (43–59) | 53 (44–61) |
| Body mass index | 0.31 | 31.9 (26.5–40.6) | 36.5 (27.7–44.4) | 31.0 (26.0–40.6) | 32.7 (26.5–39.4) |
| Diabetes mellitus | 0.19 | 169 (50%) | 31 (52%) | 52 (45%) | 86 (52%) |
| Hypertension requiring medication | 0.006 | 164 (48%) | 32 (53%) | 67 (58%) | 65 (39%) |
| Current smoker | 0.001 | 113 (33%) | 12 (20%) | 53 (46%) | 48 (29%) |
| Dependent for activities of daily living | <0.001 | n = 284 86 (30%) |
n = 7 4 (57%) |
n = 116 40 (34%) |
n = 161 42 (26%) |
| Apache II | 0.002 | 15 ( 9–24) | 13 ( 8–20) | 14 ( 9–21) | 18 ( 9–29) |
| Documented bacteremia on admission | 0.002 | 28 ( 8.1%) | 2 ( 3.3%) | 18 (15.5%) | 8 (4.8%) |
| Number of inpatient transfers | 0.007 | 145 (64%) | 47 (81%) | 41 (61%) | 57 (56%) |
| Number of people with operations at outside facility | 0.012 | n = 141 82 (58%) |
n = 46 29 (63%) |
n = 41 16 (39%) |
n = 54 37 (69%) |
Values reported as median (interquartile range) or n (%) unless otherwise stated; p: comparing sites using Kruskal-Wallis test for continuous or chi-square or Fisher exact test for nominal or categoric variables.
Many patients (30%) were not living independently before hospitalization. A significant number of patients (64%) were transferred from an outside facility as an inpatient. This was most notable at Site A, where 81% of patients were transferred from another facility. More than half of all transfers (58%) had at least one operation at an outside facility. Inpatient transfer rates did differ significantly between the three facilities, ranging 56%–81% of NSTI admissions. Bacteremia at admission was significantly more frequent at Site B than the other two sites. The APACHE II score was significantly higher in Site C than the other two sites.
Hospital course
There were significant practice variations (Table 2) regarding the number of antibiotic days for treatment of patients with NSTI, with Site C patients receiving the fewest days of antibiotic therapy (median 9 d, IQR: 6-14). No difference was noted among centers in median time to starting empiric antibiotics (day 1, interquartile range [IQR]: 1–1, range 1–9), with most antibiotics initiated near the time of hospital admission.
Table 2.
Hospital Course for All Sites*
| Variable | p | Total n = 341 | Site A n = 60 | Site B n = 116 | Site C n = 165 |
|---|---|---|---|---|---|
| Total number of operations | 0.001 | 2 ( 2–4) | 2 ( 1–3) | 2 (1–3) | 3 ( 2–4) |
| Number of patients requiring amputation | 0.13 | 40 (12%) | 3 ( 5%) | 11 (9%) | 26 (16%) |
| Total number of surgical debridements | 0.10 | 2 ( 1–3) | 2 ( 1–2) | 2 (1–2) | 2 ( 1–3) |
| Time to initiation of antibiotics from admission (d) | 0.70 | 1 ( 1–1) | 1 ( 1–1) | 1 (1–1) | 1 ( 1–1) |
| Number of antibiotic days | <0.001 | n = 335 | n = 59 | n = 114 | n = 162 |
| 11 ( 7–19) | 16 (10–28) | 12 (8–18) | 9 ( 6–14) |
Values reported as median (interquartile range) or n (%) unless otherwise stated; p: comparing sites using Kruskal-Wallis test for continuous or chi-square or Fisher exact test for nominal or categorical variables.
On admission to a study center, most patients had an operation on hospital day one (IQR 1–2). The median number of surgical debridements was two (IQR 1–3), and we found no significant difference between centers regarding total number of surgical debridements, time from admission to first operation (n = 0.40), and rate of purulence noted at first operation (n = 0.07), nor in amputation rate. Rate of post-operative complications varied among centers, with differences noted in the rates of UTI but not in rates of hospital-acquired pneumonia. Nine patients had documented Clostridium difficile infections—one at Site A (1.7% incidence), five at Site B (4.3% incidence), and three at Site C (1.8% incidence).
In survivors, the median LOS was 19 d (IQR: 12–31), and site comparison showed significant variation in LOS (Table 3). More than half of all survivors (n = 290, 53%) were discharged to home with or without home health, with site differences between rates of discharge home (Table 3). A total of 99 patients required re-admission; of these, 47% (47/99) patients were unplanned re-admissions, mostly because of surgical site infection or disruption. There was no significant difference noted between sites regarding re-admission overall or unplanned re-admission.
Table 3.
Outcomes and Complications*
| Variable | p | Total n = 341 | Site A n = 60 | Site B n = 116 | Site C n = 165 |
|---|---|---|---|---|---|
| Post-operative complications | <0.001 | 163 (48%) | 41 (68%) | 71 (61%) | 51 (31%) |
| Pneumonia | 0.49 | 25 ( 7%) | 3 ( 5%) | 11 ( 9%) | 11 ( 7%) |
| Urinary tract infection | 0.02 | 48 (14%) | 15 (25%) | 16 (14%) | 17 (10%) |
| Mortality rate | 0.02 | 49 (14%) | 13 (22%) | 21 (18%) | 15 ( 9%) |
| Length of stay** | 0.001 | 19 (12–31) | 29 (19–67) | 16 (10–24) | 19 (12–33) |
| Discharged home** | 0.01 | n = 292 | n = 47 | n = 95 | n = 150 |
| 153 (53%) | 27 (57%) | 47 (49%) | 79 (53%) |
Values reported as median (interquartile range) or n (%) unless otherwise stated; p: comparing sites using Kruskal-Wallis test for continuous or chi-square or Fisher exact test for nominal or categorical variables;**survivors only.
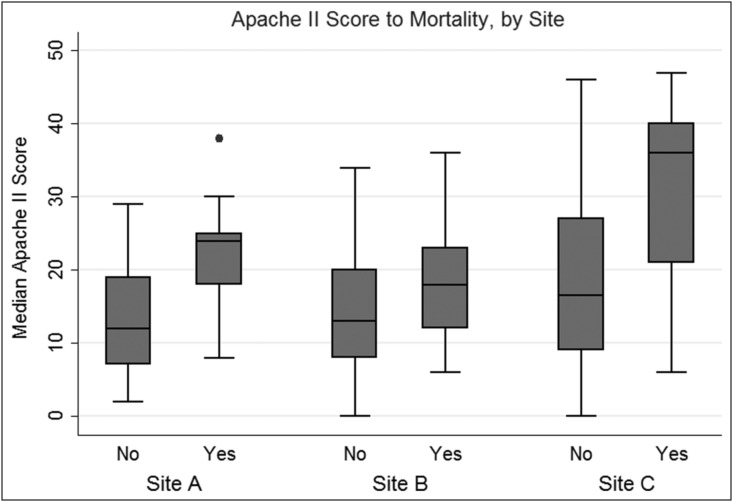
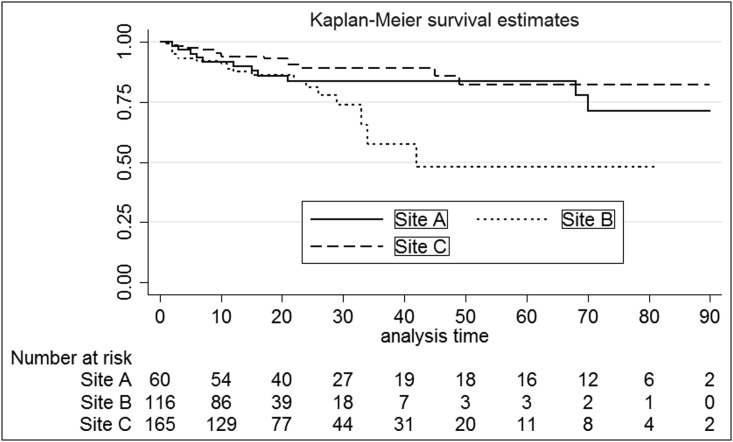
The overall mortality rate was 14% and differed significantly between centers. Figure 1 shows the trend in APACHE II severity compared with death by site, illustrating that Site C had the highest severity of illness based on Apache II with the lowest mortality rate of the three centers. When comparing survivors versus non-survivors, neither number of antibiotic days nor extended time to antibiotic start impacted death. Inpatient transfers did not achieve a significant impact on death, with Site A having the highest mortality rate (22%) and highest inpatient transfer rate (81%). Figure 2 shows the markedly different Kaplan-Meier survival estimates and corresponding risk table. The risk table breaks down the number of patients by site over LOS up to 90 days.
FIG. 1.
Trend in Apache II severity scores compared with death by site.
FIG. 2.
Markedly different Kaplan-Meier survival estimates and corresponding risk table by site. The risk table breaks down the number of inpatients at a specific day by site up to 90 days.
We created several multivariable logistic regression models to examine risk factors for death as a result of NSTI. Both transfer from another acute care facility (p = 0.66) and admission Sequential Organ Failure Assessment score (p = 0.22–0.89 for quartiles analyzed) failed to achieve significance and were dropped from subsequent models; the same was found for functional status, which narrowly missed significance in the multi-variable model (p = 0.056). Our best model is shown in Table 4, and included APACHE score by quartile, antibiotic days, and care site as significant predictors of death.
Table 4.
Multi-Variable Logistic Analysis
| Independent variable | Odds ratio | se | z ratio | Prob. | 95% CI | |
|---|---|---|---|---|---|---|
| Antibiotic days (quartiles) | ||||||
| 8–11 d | 0.252 | 0.130 | −2.65 | 0.008 | 0.091 | 0.698 |
| 12–18 d | 0.162 | 0.090 | −3.28 | 0.001 | 0.055 | 0.481 |
| 19–185 d | 0.313 | 0.136 | −2.68 | 0.007 | 0.134 | 0.732 |
| Site | ||||||
| A | 6.50 | 3.40 | 3.58 | <0.001 | 2.33 | 18.096 |
| B | 5.35 | 2.33 | 3.84 | <0.001 | 2.27 | 12.587 |
| APACHE II | 1.09 | 0.020 | 4.71 | <0.001 | 1.053 | 1.133 |
| Constant | 0.025 | 0.015 | −6.26 | <0.001 | 0.008 | 0.080 |
Model χ2 = 48.68.
Pseudo R2 = 0.177.
n = 335.
CI = confidence interval; APACHE = Acute Physiology and Chronic Health Evaluation.
Referents for independent variables were 1–7 days for antibiotics and Site C.
The dependent variable in this model is death resulting from necrotizing soft-tissue infections, coded so that 0 = lived and 1 = died.
Discussion
NSTI is relatively uncommon but are associated with a high burden of surgical and critical care, as well as significant morbidity and mortality for patients who have them. Surgical debridement and broad-spectrum empiric antibiotics are first-line therapy for NSTI, but practice guidelines for best management of NSTI after initial antibiotics and debridement do not exist. Our study illustrates variation in clinical practices at several institutions, such as antibiotic commencement and duration. Our findings are consistent with the implication of Kao et al. [6] that practice variation may be a driver of outcome variance in NSTI, particularly practices related to antibiotic stewardship. Alternatively, microbial patterns may impact outcome variance, although this topic is beyond the scope of the current study.
Previous research has shown that morbidity and mortality increase when patients are transferred from outside facilities [2,3,7]. Site A had the highest mortality rate (22%), and the site also had the highest rate of transfers (81%). Unfortunately, time from illness presentation to hospital admission was not captured, nor was antibiotic administration or type of surgical procedure at the outside facility captured. Site B and Site C did have similar rates of operations at outside facilities. A key limitation was that we were unable to obtain information about antibiotic management and operative details of patient management before transfer to a study center.
One of the most provocative findings of this review is that an extended duration of antibiotic therapy did not translate to better outcomes in the patient population studied. Past studies have documented median antibiotic use of approximately 13 days or suggested 10–14 days of use [1,13]. Death associated with NSTI was lowest at Site C, however, where the median treatment time was nine antibiotic days. The nosocomial infection rate at Site C also showed a trend toward being lower than at the other two centers.
The total number of operations was significantly different (p < 0.001) between treatment centers, but the number of surgical debridements was not. The rate of amputation in our study was 12%, with wide variability between the three centers. Previous studies have shown overall amputation rates ranging 10%–26% [8,13,14] Site A did have a lower rate of amputation than the other two centers and those reported in the literature. This limb-conserving approach may account for their longer duration of supportive care because of more challenging intensive care unit stays.
Outcomes among centers were significantly different with regard to mortality rate and LOS. Previous studies have yielded mortality rates ranging 13%–32% [8,9,14–16]. Endorf et al. [17] showed that death from NSTI at verified burn centers is higher than that of non-burn centers, likely because of transfer patterns and the increased acuity observed at burn centers. The overall mortality rate observed in this study was 14%. Among the study centers, Site C's mortality rates were significantly lower than those at the other centers. This difference could not be accounted for by bacteremia on admission or APACHE II scores and thus may be reflective of practice variation in either antibiotic or operative management. With regard to LOS, Site B showed the shortest LOS among centers. The overall observed median LOS was 19 days, shorter than a previously reported LOS of 24 days in a similar cohort [13].
The current study is limited by its retrospective nature and sampling of only three hospitals with burn centers within the United States. We had no metric for adequacy or aggressiveness of surgical debridement, which may be a driver of shorter antibiotic duration and lower deaths simply because of the importance of source control in NSTI treatment. We were unable to differentiate monomicrobial from polymicrobial infections based on the data collected, and therefore were unable to adjust for outcome differences based on the type of NSTI. We also do not have data that allow us to assess type of antibiotics used based on culture results, and we therefore cannot make any inferences about the appropriateness of antibiotic coverage.
In addition, the study was not sufficiently powered to determine how varying practices translate to patient outcomes in the context of such a high number of variables. We were unable to obtain data on specific time of initiation of antibiotics because of the retrospective nature of this study, something that is a notable limitation in terms of early therapy for this patient population; this also means that our data on time to antibiotics and time to operation, both of which were measured in days rather than hours, may not be indicative of true differences in practice. Also, Site A had far fewer patients than the other two sites, meaning that all sites are not equally represented in the data set. The significantly increased proportion of transfer patients at one site could also impact the generalizability of the results, and clearly accounted for some of the noted practice variation. Unfortunately, we did not have access to comprehensive initial patient presentation and management data on those patients transferred from outside facilities.
Using data from three centers with varying clinical practices, we have an opportunity to compare differences in aspects of NSTI treatment patterns and their associated outcomes. These findings demonstrate there remain very different practice patterns in the treatment of patients with NSTI, even at centers that have clinical expertise in this disease; this study is the first to indicate that limiting the duration of antibiotic management in NSTI may be positively associated with survival.
In general, the outcomes observed in the current cohort are favorable when compared with the literature, but many questions remain if we are to improve quality of care and clinical outcomes in this heterogeneous patient population. Development of a clinical practice improvement study with more granular data collection on antibiotic initiation, bacterial sensitivities, and practices of antibiotic narrowing in response to cultures would provide a foundation for development of practice-based evidence on a more broad level, and represents an important next step in improving the clinical science of NSTI care.
Acknowledgments
The Center for Clinical and Translational Sciences at the University of Utah received grant support (8UL1TR000105 (formerly UL1RR025764) NCATS/NIH) for REDCap.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing fasciitis: Current concepts and review of the literature. J Am Coll Surg 2009;208:279–288. http://www.sciencedirect.com/science/article/pii/S1072751508015469 (last accessed October7, 2016) [DOI] [PubMed]
- 2.Mills MK, Faraklas I, Davis C, et al. Outcomes from treatment of necrotizing soft-tissue infections: Results from the National Surgical Quality Improvement Program database. Am J Surg 2010;200:790–796 [DOI] [PubMed] [Google Scholar]
- 3.Holena DN, Mills AM, Carr BG, et al. Transfer status: A risk factor for mortality in patients with necrotizing fasciitis. Surgery 2011;150:363–370 [DOI] [PubMed] [Google Scholar]
- 4.Stevens DL, Bis no AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373–1406 [DOI] [PubMed] [Google Scholar]
- 5.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003;290:2588–2598 [DOI] [PubMed] [Google Scholar]
- 6.Kao LS, Lew DF, Arab SN, et al. Local variations in the epidemiology, microbiology, and outcome of necrotizing soft-tissue infections: A multicenter study. Am J Surg 2011;202:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggerstedt M, Gamelli RL, Mosier MJ. The care of necrotizing soft-tissue infections: Patterns of definitive intervention at a large referral center. J Burn Care Res 2015;36:105–110 [DOI] [PubMed] [Google Scholar]
- 8.Anaya DA, Bulger EM, Kwon YS, et al. Predicting death in necrotizing soft tissue infections: A clinical score. Surg Infect 2009;10:517–522 [DOI] [PubMed] [Google Scholar]
- 9.Faraklas I, Stoddard GJ, Neumayer LA, Cochran A. Development and validation of a necrotizing soft-tissue infection mortality risk calculator using NSQIP. J Am Coll Surg 2013;217:153–160 [DOI] [PubMed] [Google Scholar]
- 10.Bulger EM, Maier R V, Sperry J, Joshi M, Henry S, Moore FA, et al. A novel drug for treatment of necrotizing soft-tissue infections: A randomized clinical trial. JAMA Surg 2014;149:528–536 [DOI] [PubMed] [Google Scholar]
- 11.Brown DR, Davis NL, Lepawsky M, et al. A multicenter review of the treatment of major truncal necrotizing infections with and without hyperbaric oxygen therapy. Am J Surg 1994;167:485–489 [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernal NP, Latenser BA, Born JM, Liao J. Trends in 393 necrotizing acute soft tissue infection patients 2000–2008. Burns 2012;38:252–260 [DOI] [PubMed] [Google Scholar]
- 14.McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 1995;221:558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg 2000;179:361–366 [DOI] [PubMed] [Google Scholar]
- 16.Gunter OL, Guillamondegui OD, May AK, Diaz JJ. Outcome of necrotizing skin and soft tissue infections. Surg Infect 2008;9:443–450 [DOI] [PubMed] [Google Scholar]
- 17.Endorf FW, Klein MB, Mack CD, et al. Necrotizing soft-tissue infections: Differences in patients treated at burn centers and non-burn centers. J Burn Care Res 2008;29:933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]