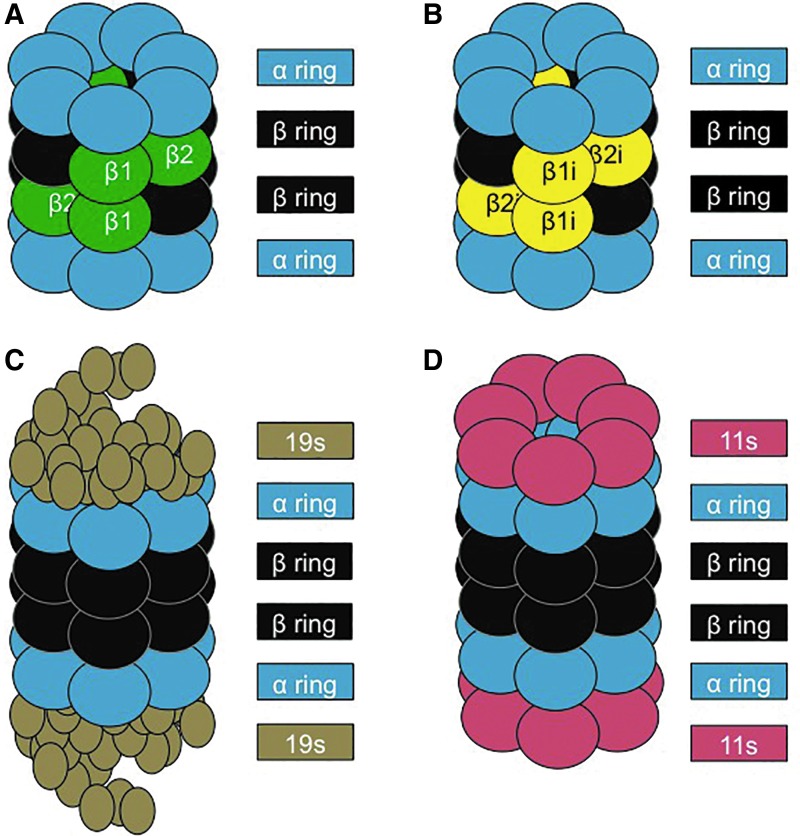
FIG. 1.
Structure of the proteasome and its regulatory subunits. (A) The 20S core proteasome is formed by four rings. The two outer rings contain seven α subunits (1–7), and the two inner rings consist of seven β subunits (1–7). Proteolysis is catalyzed within the β rings by three distinct β subunits: β1, β2, and β5. (B) The IP differs from the 20S proteasome by having three unique proteolytic sites: β1i, β2i, and β5i, which allow different substrate processing and larger peptide products that can be utilized in MHC class I antigen presentation. (C) The 26S Proteasome is formed on the addition of two 19S regulators (each consisting of multiple subunits) to the α rings of the 20S core Proteasome, allowing the 26S Proteasome to recognize and unfold (using ATP) poly-ubiquitin-tagged substrates for degradation. (D) 11S or Pa28 is a regulator that can bind to the α rings of both the 20S Proteasome and the IP, to enhance the degradation of oxidatively damaged protein substrates. ATP, adenosine triphosphate; IP, Immunoproteasome. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars