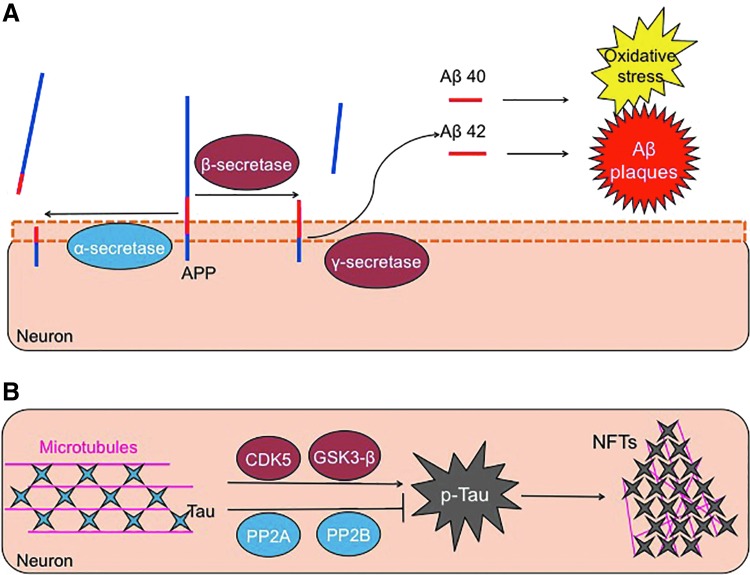
FIG. 3.
Aβ and p-Tau formation. (A) The APP is a transmembrane protein. APP can be cleaved in a nonpathological way through the action of an α-secretase, an enzyme that breaks the Aβ sequence. Conversely, APP can be cleaved in a pathological manner, involving a first cut by a β-secretase, followed by a second hydrolysis by a γ-secretase. These two cleavage steps form two slightly different Aβ peptides, which, in turn, can aggregate to form amyloid plaques and also cause much of the oxidative stress related to this disease. The two Aβ isoforms are 40 and 42 amino acids length (Aβ1-40 and Aβ1-42), with Aβ1-42 being, by far, the most toxic. (B) The Tau protein helps to maintain the structure of microtubules. The action of some kinases such as cyclin-dependent kinase 5 (CDK5) and GSK3β phosphorylates Tau. If Tau becomes excessively phosphorylated, or hyperphosphorylated, it tends to form aggregates that are known as NFTs. This process is also regulated by dephosphorylating enzymes, such as phosphatase 2A and 2B, that act to remove excessive phosphoryl groups and help Tau keep its proper structure and functionality. Aβ, amyloid-β; APP, amyloid precursor protein; GSK3β, glycogen synthase kinase 3β; NFTs, neurofibrillary tangles. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars