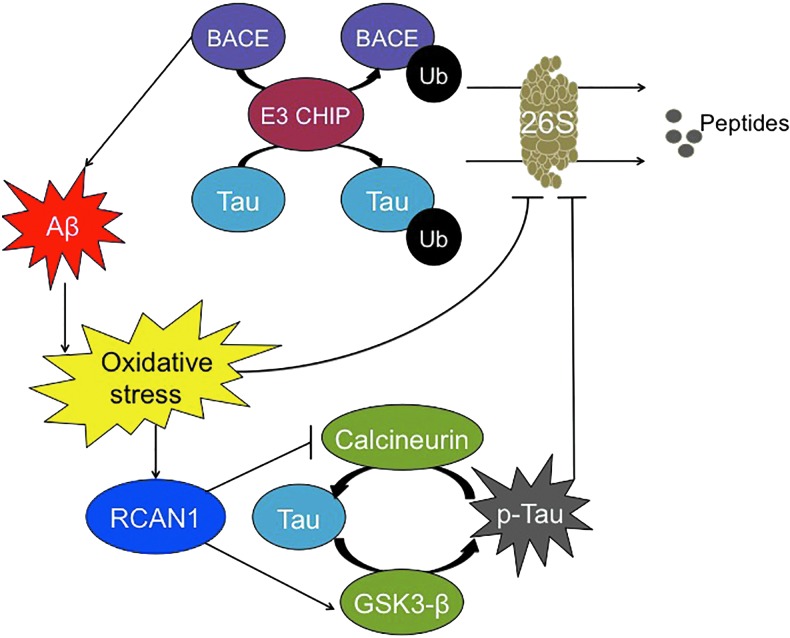
FIG. 5.
Relationship between Aβ and Tau. BACE-1, responsible for Aβ formation, and the Tau protein can be ubiquitinylated by the E3 CHIP ligase and degraded by the 26S Proteasome. However, Aβ aggregation inhibits 26S Proteasome activity indirectly, due to increased oxidative stress. Simultaneously, oxidative stress induces RCAN1 synthesis, which blocks calcineurin activity, causing decreased dephosphorylation of Tau, and it stimulates GSK3β activity, causing increased Tau phosphorylation: The net result is a large net increase in the steady-state phosphorylation of Tau, which then cannot be degraded by Proteasome. CHIP, C terminus of heat shock protein 70 interacting protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars