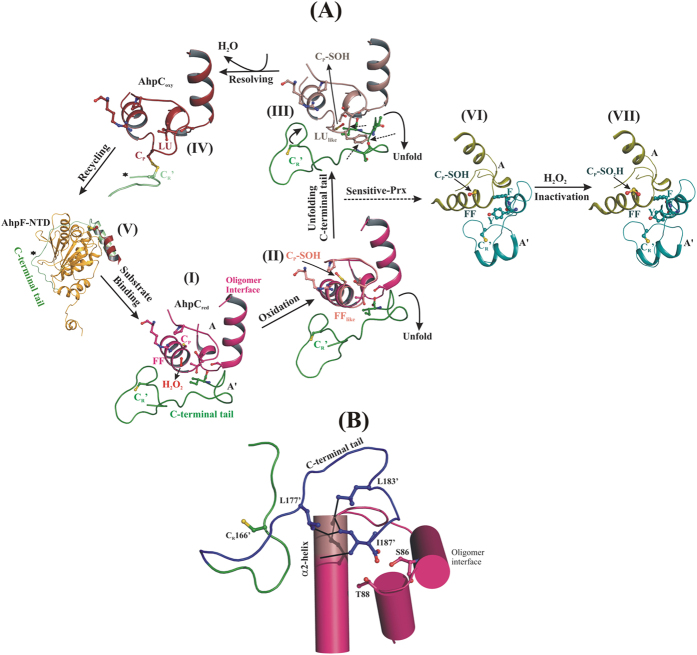
Figure 10. The proposed transition state(s) during the catalytic cycle of Prxs.
(A) (I) The reduced CP adopts FF conformation and is essential to activate and stabilize the CP-thiolate for peroxidation. (II) The oxidative modification of CP to CP-SOH leads to localized structural alterations around the conserved active site pocket as seen from the “FFlike” conformation of the reduced EcAhpC1-186-YFSKHN. (III) The “LUlike” conformation in the reduce EcAhpC1-186-YFSKHN structure, reveals a severe clash between the active site residues with the C-terminal tail. The entire C-terminal arm (aa 166–187) is needed to undergo alterations and to enable the disulphide formation between CR’ and CP (solid arrow). (IV) The disulphide bonded active site is stabilized in the LU conformation, wherein the C-terminal tail is disordered (denoted by asterisk). (V) The C-terminal tail of AhpC binds to its reducing partner, the N-terminal domain (NTD) of AhpF, to regenerate for another catalytic cycle. (VI) In human Prxs, the extra C-terminal YFSKHN-helix is folded across the active site, thereby delaying the FF to LU transition during the intermediate CP-SOH state. In such case, the CP-SOH form can further react with peroxide and leads to enzyme inactivation (VII). (B) The cartoon representation shows that the CP and the first turn of the α2-helix is stabilized by the weak interactions with the I187 and C-terminal tail, which is hold by residues S86 and T88. Disruption of weak interactions of the C-terminal tail, like between I187 and the α2-helix or between I187 with S86A and T88A, leads to unfolding of the C-terminal tail from the active site region.