Abstract
Pancreas development requires restrained Hedgehog (Hh) signaling activation. While deregulated Hh signaling in the pancreatic mesenchyme has been long suggested to be detrimental for proper organogenesis, this association was not directly shown. Here, we analyzed the contribution of mesenchymal Hh signaling to pancreas development. To increase Hh signaling in the pancreatic mesenchyme of mouse embryos, we deleted Patched1 (Ptch1) in these cells. Our findings indicate that deregulated Hh signaling in mesenchymal cells was sufficient to impair pancreas development, affecting both endocrine and exocrine cells. Notably, transgenic embryos displayed disrupted islet cellular composition and morphology, with a reduced β-cell portion. Our results indicate that the cell-specific growth rates of α- and β-cell populations, found during normal development, require regulated mesenchymal Hh signaling. In addition, we detected hyperplasia of mesenchymal cells upon elevated Hh signaling, accompanied by them acquiring smooth-muscle like phenotype. By specifically manipulating mesenchymal cells, our findings provide direct evidence for the non-autonomous roles of the Hh pathway in pancreatic epithelium development. To conclude, we directly show that regulated mesenchymal Hh signaling is required for pancreas organogenesis and establishment of its proper cellular composition.
The pancreas comprises of exocrine and endocrine cell populations with defined proportions and distinct functions in food digestion and blood glucose regulation, respectively. The majority of pancreatic tissue is populated by exocrine cells, primarily acinar cells, while endocrine cells are organized in islets of Langerhans that are embedded in the exocrine tissue1. Islets are composed of α-, β-, δ-, and polypeptide-positive cells, where the predominant cell population is that of β-cells2. Pancreas cellular composition is largely dictated during organogenesis, through tightly-regulated cell-specific differentiation and growth rates3,4,5,6. These are determined by a combination of intrinsic and extrinsic cues, and are therefore highly dependent on interactions between neighboring cells3,6,7,8. Indeed, pancreas organogenesis requires proper interactions of the developing epithelium with its surrounding mesenchyme7,8,9,10,11,12. Although evidence on the importance of the pancreatic mesenchyme in supporting epithelial cell growth and differentiation is accumulating, the question as to whether or not a differential response to mesenchymal cues determines pancreas cellular composition remains open.
During pancreas organogenesis, mesenchymal cells support various developmental stages. The pancreatic epithelium forms from the embryonic foregut endoderm, which recruits adjacent splanchnic mesoderm to form the mesenchymal compartment of the developing organs3. At the onset of organogenesis (in mice, around embryonic day (e) 9), the pancreatic bud is populated by common precursors that require mesenchymal cues for their proliferation and survival7,8,9. Later in development (around mouse e12.5–e14.5), as these cells become committed to either an exocrine or endocrine fate1,13,14, their proliferation and maintenance was shown to depend on mesenchymal cues10,11,12. Finally, we previously showed that toward the end of gestation (mouse e16.5–e18.5), the pancreatic mesenchyme supports proliferation of differentiated epithelial cells, including β-cells11. However, whether mesenchymal cues differentially affect the distinct epithelial cell types awaits further investigation.
Regulated Hedgehog (Hh) signaling pathway is required for proper pancreas organogenesis15,16. Activation of this pathway is dependent on the binding of a Hh ligand to its transmembrane receptor, Patched (Ptch)17,18. The binding of each of the secreted Hh ligands (Sonic (Shh), Indian (Ihh) and Desert (Dhh) hedgehog) releases the repression that Ptch puts on Smoothened (Smo), allowing nuclear localization of the Gli family of transcription factors, and in turn results in expression of Hh-dependent genes17. Of note, one of these Hh-dependent genes is Ptch1, allowing a negative feedback loop on this pathway17. While Hh ligands are highly expressed in adjacent developing organs, namely the stomach and guts, they are absent from the pancreatic epithelium at early stages of development and found in low levels at later stages19,20,21,22. Enforced activation of the Hh pathway in the developing pancreas, in mice lacking regulatory elements23,24 or ectopically expressing Hh ligands in the pancreatic epithelium19,25, resulted in tissue agenesis, pointing to the essential role of tightly-regulated Hh signaling in pancreas development.
Disrupted Hh signaling affected the development of both endocrine and exocrine cells, and was particularly shown to impair β-cell function and mass23,24. To differentiate between Hh signaling activation in the pancreas epithelium and its mesenchyme, transgenic mice, in which this pathway was specifically manipulated in the pancreatic epithelium, were generated22,26,27. Regulated Hh signaling within the pancreatic epithelium was shown by others and by us to be required for proper endocrine mass and β-cell function26,27. Of note, these epithelial-specific manipulations did not fully recapitulate the developmental phenotype observed upon pancreatic-wide manipulation of this pathway19,23,24,25. Indeed, it has been long suggested that regulated Hh signaling in the pancreatic mesenchyme is required for proper development19. However, the requirement of regulated mesenchymal Hh signaling for pancreas development was not directly shown.
Here, we specifically manipulated pancreatic mesenchymal Hh signaling and studied the resultant effects on epithelial development, focusing on the endocrine pancreas. To this end, we combined the use of Ptch1flox mice, which allows Cre-dependent deletion of this gene28, with Nkx3.2-Cre11,29, expressed by the pancreatic mesenchyme, to generate embryos with elevated levels of Hh signaling in their pancreatic mesenchyme. We observed reduced pancreatic mass in transgenic embryos, when both endocrine and exocrine area were reduced. Interestingly, cellular composition of the endocrine pancreas was disturbed in these embryos when β-cell proportion decreased, indicating it is regulated by Hh-sensitive mesenchymal activity. We further detected hyperplasia of the pancreatic mesenchyme in transgenic embryos, accompanied by abnormal αSMA (α Smooth Muscle Actin) expression. By specifically manipulating mesenchymal cells, our findings provide direct evidence for the non-autonomous roles of the Hh pathway in pancreatic epithelium development.
Results
Increased Hh signaling in the embryonic pancreatic mesenchyme upon Ptch1 deletion
To manipulate Hh signaling in the pancreatic mesenchyme, we set to inhibit the expression of Ptch1, a negative regulator of Hh signaling transduction, which its deletion was shown to increase expression of target genes17. To this end, we generated Nkx3.2-Cre;Ptch1flox/flox embryos, in which the two copies of Ptch1 are deleted in the Nkx3.2-Cre lineage in a Cre/lox dependent manner28,29. We have previously shown that the Nkx3.2-Cre line allows for manipulation of mesenchymal cells, but for no other cell types (including epithelial, endothelial and neuronal) in the developing pancreas11. To assess the level of Hh signaling activation, we analyzed the expression levels of Gli1, a target gene of this pathway, in the embryonic pancreas of Nkx3.2-Cre;Ptch1flox/flox and non-transgenic control littermates (Ptch1flox; Cre-negative) at e14.5. Our analysis revealed increased expression of Gli1 in pancreatic tissue of transgenic embryos (Fig. 1A), indicating elevated levels of Hh signaling.
Figure 1. Deletion of Ptch1 using Nkx3.2-Cre mouse line activates Hh signaling in the pancreatic mesenchyme.
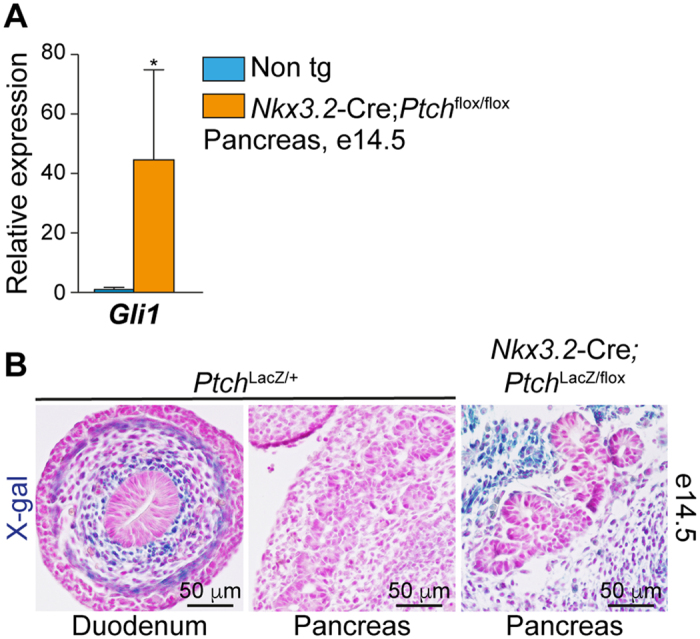
(A) Bar diagram showing Gli1 expression levels in pancreatic tissues from Nkx3.2-Cre;Ptchflox/flox (orange bar) and non-transgenic littermate controls (‘non tg’; cyan bar) at embryonic day (e)14.5. Tissues were dissected, their RNA isolated, and gene expression levels were analyzed by qPCR and normalized to non-transgenic controls (n = 3–4). *P < 0.05 (Student’s t test). Data represent mean ± SD. (B) Dissected gut and pancreatic tissues at embryonic day (e)14.5 incubated with X-gal (blue) and counterstained with Fast Red (pink). Shown are PtchLacZ/+ duodenum (left panel) and pancreatic tissue (middle panel), and Nkx3.2-Cre;PtchLacZ/flox pancreatic tissue (right panel).
To assess the localization of Hh signaling activation, we monitored Ptch1 expression in the embryonic pancreas. As Ptch1 is a Hh signaling target gene, Ptch1LacZ mouse line, in which a copy of this gene was knocked in by a LacZ cassette, serves as a reporter for Hh signaling activation30. In Ptch1LacZ e14.5 embryos, LacZ activity is abundant in the mesenchyme surrounding the developing duodenum epithelium (Fig. 1B). However, despite the removal of one copy of Ptch1, and in agreement with previous studies22,24, this activity was below detection in the pancreatic mesenchyme (Fig. 1B). Next, we generated Nkx3.2-Cre;Ptch1LacZ/flox compound embryos, in which one copy of Ptch1 was knocked in by the LacZ transgene, and the other (Ptch1flox) depleted in the Nkx3.2-Cre lineage, as described above. Analysis of LacZ activity in Nkx3.2-Cre;Ptch1LacZ/flox e14.5 embryos pointed to increased Hh signaling in their pancreatic mesenchyme, but not in the epithelium (Fig. 1B). Thus, deleting Ptch1 using the Nkx3.2-Cre mouse line allowed increased mesenchymal Hh signaling in the developing pancreas.
Deletion of Ptch1 in the pancreatic mesenchyme results in reduced pancreatic mass
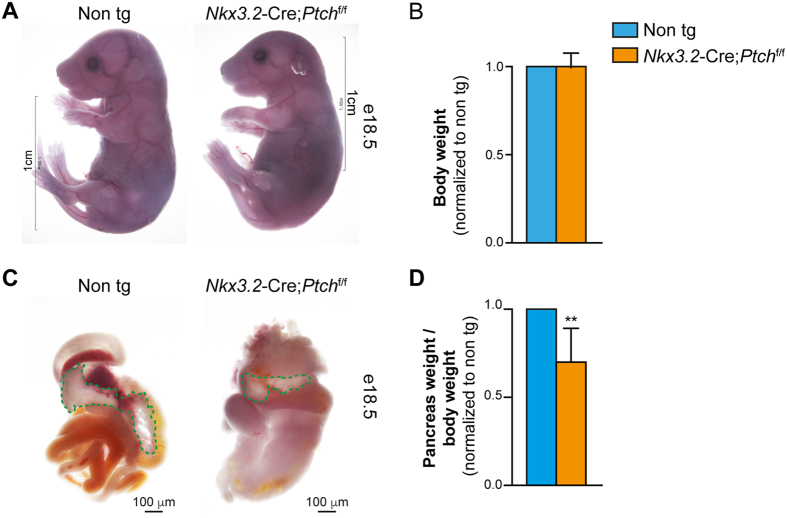
To analyze the resultant effect of increased mesenchymal Hh signaling on pancreatic development, we analyzed Nkx3.2-Cre;Ptch1flox/flox embryos and non-transgenic control littermates (Ptch1flox; Cre-negative). Nkx3.2-Cre;Ptch1flox/flox mice died upon birth, likely due to non-pancreatic expression of the Nkx3.2-Cre line in the embryonic gut and stomach mesenchyme and in skeletal somites29,31. However, at e18.5 transgenic embryos exhibited comparable appearance and body weight to their non-transgenic control littermates (Fig. 2A and B). As previously reported upon elevated Hh signaling in the Nkx3.2-Cre lineage32, the gastrointestinal tract of transgenic mice was drastically deformed, with short and dilated intestine and misshapen stomach (Fig. 2C). Of note, we were unable to detect splenic tissue, a derivative of the embryonic pancreatic mesenchyme33, in transgenic mice. Nonetheless, pancreatic tissue could be detected in Nkx3.2-Cre;Ptch1flox/flox embryos (Fig. 2C). Pancreatic tissue of transgenic embryos was significantly smaller than that of their non-transgenic littermates (Fig. 2D), implicating that increased Hh signaling in the pancreas mesenchyme affects proper organogenesis.
Figure 2. Reduced pancreatic mass upon increased mesenchymal Hh signaling.
Nkx3.2-Cre;Ptchflox/flox transgenic and non-transgenic (Cre negative; ‘Non tg’) littermate embryos were analyzed at e18.5. (A) Whole body images of transgenic embryo (right) and non-transgenic littermate (left). (B) Bar diagram (mean ± SD) summarizing normalized body weight of transgenic (orange bar) to non-transgenic (cyan bar; set to ‘1’) littermates. n = 5. (C) Images show gross morphology of dissected embryonic gastrointestinal tract from transgenic (right) and non-transgenic (left) littermates. Pancreatic tissue is outlined with a green dashed line. (D) Bar diagram (mean ± SD) summarizing normalized pancreas weight of transgenic (orange bar) to non-transgenic (cyan bar; set to ‘1’) littermates. n = 5. p value: **P < 0.01, as compared to non-transgenic control, determined using Student’s t-test.
Elevated Hh signaling leads to expansion of the pancreatic mesenchyme
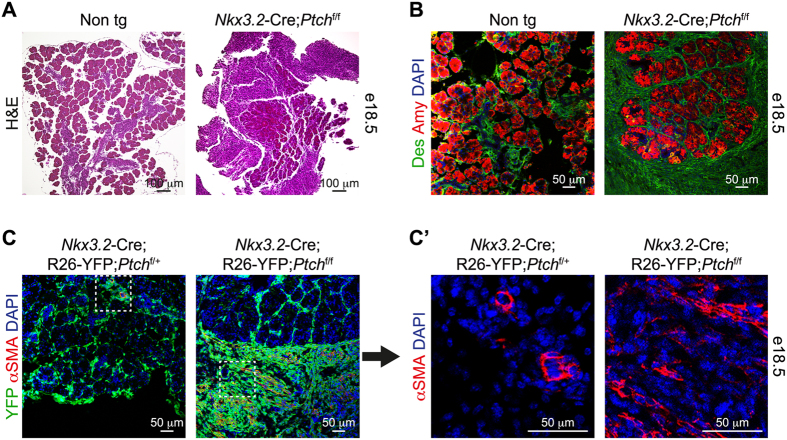
To further analyze the effect of deregulated mesenchymal Hh signaling on pancreas development, we dissected pancreatic tissue from e18.5 Nkx3.2-Cre;Ptch1flox/flox and non-transgenic littermate control embryos. While non-transgenic pancreatic tissue contained mostly epithelial cells, histological analysis revealed abundant non-epithelial cells in pancreatic tissue of Nkx3.2-Cre;Ptch1flox/flox embryos (Fig. 3A).
Figure 3. Hyperplasia of the pancreatic mesenchyme upon elevated Hh signaling.
(A) Histological analysis of pancreatic tissue of e18.5 Nkx3.2-Cre;Ptchflox/flox transgenic (right) and non-transgenic (Cre negative; ‘Non tg’; left) littermate embryos. Tissue-sections stained with Hematoxylin and Eosin (H&E). (B) Pancreatic tissues of e18.5 Nkx3.2-Cre;Ptchflox/flox transgenic and non-transgenic (Cre negative; ‘Non tg’) littermate embryos were stained with antibodies against Desmin (‘Des’; green) and Amylase (‘Amy’; red) and counterstained with DAPI (blue). Shown are representative fields. (C) Immunofluorescence analysis of dissected pancreatic tissues from Nkx3.2-Cre;R26-YFP;Ptchflox/flox (right) and Nkx3.2-Cre;R26-YFP;Ptchflox/+ (left) control littermate e18.5 embryos. Tissues were stained with antibodies against YFP (green), α smooth muscle actin (αSMA; red) and counterstained with DAPI (blue). C’) Higher magnification of areas framed in a white box in (C) showing the αSMA and DAPI channels. Shown are representative fields.
Hh signaling was shown to support proliferation of mesenchymal cells lining the developing gastrointestinal tract32. In order to test if elevated Hh signaling leads to changes in the mesenchymal cell layer, we labeled these cells using the pan-mesenchymal marker desmin. In control embryos, desmin-expressing cells formed a thin, cell-wide layer surrounding acinar lobes (Fig. 3B). In contrast, transgenic pancreatic tissues displayed multi-cellular desmin-positive mesenchymal layer that extended away from the epithelium (Fig. 3B). Of note, expanded mesenchymal layer was detected also in between acinar cells. Thus, our results point to hyperplasia of the pancreatic mesenchyme upon increased Hh signaling in these cells.
Pancreatic mesenchymal cells were shown to acquire smooth muscle fate, typical to mesenchymal cells surrounding the gut, in response to increased Hh signaling19. To study potential changes in these cells, we employed the R26R-YFP transgenic mouse line, which allows for YFP expression in a Cre-dependent manner34. As Nkx3.2-Cre express mesenchymal cells from early stages of pancreas development11,31, the inclusion of a Cre-dependent YFP reporter allowed us to trace these cells, regardless of potential phenotypical changes. To analyze for potential acquisition of smooth muscle fate upon elevated mesenchymal Hh signaling, we analyzed the expression of αSMA in YFP-expressing cells. As expected, in control Nkx3.2-Cre;R26R-EYFP;Ptch1flox/+ pancreatic tissue, αSMA expression was observed in vascular smooth muscle cells (vSMCs), embedded in the pancreatic tissue (Fig. 3C and C’)35. However, in Nkx3.2-Cre;R26R-EYFP;Ptch1flox/flox embryos, this marker was expressed by YFP-expressing cells that extended away from the pancreatic epithelium in transgenic embryos (Fig. 3C and C’). Furthermore, the fusiform shape of αSMA-expressing cells (Fig. 3C’) further suggests pancreatic mesenchymal cells acquire smooth muscle-like phenotype upon deregulated Hh signaling, as previously suggested19.
To conclude, in agreement with previous reports19,32, we observed hyperplasia of the pancreatic mesenchyme upon elevated Hh signaling, accompanied by acquisition of smooth muscle-like phenotype.
Reduced epithelial area upon deletion of Ptch1 in the pancreatic mesenchyme
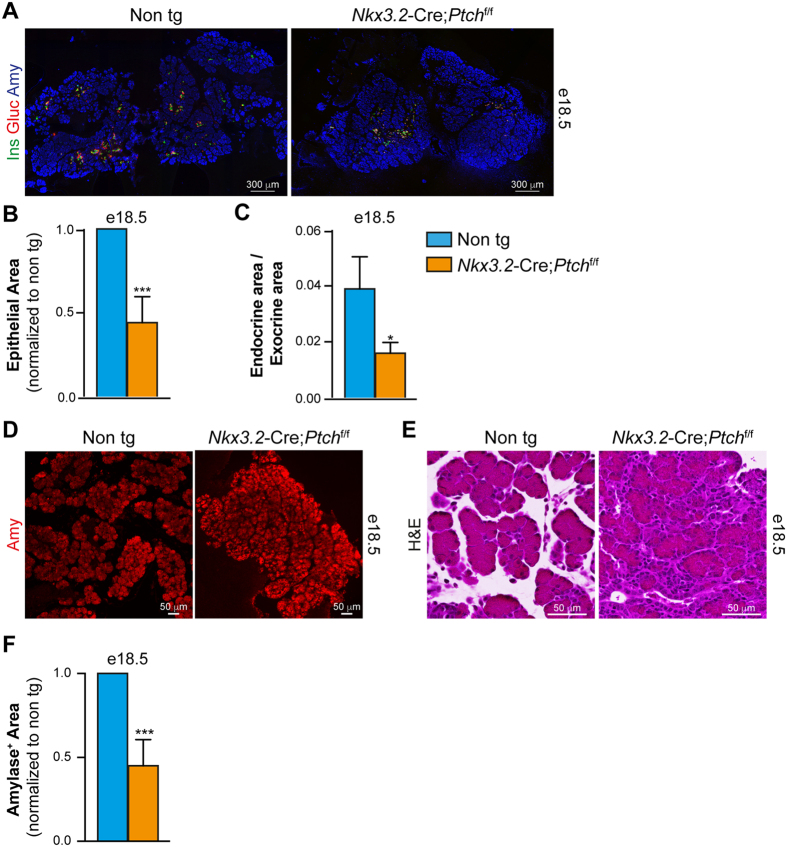
To analyze the resultant effect of increased mesenchymal Hh signaling on pancreatic epithelial development, we analyzed for the presence of the most abundant pancreatic epithelial cell types, β-, α- and acinar cells, in Nkx3.2-Cre; Ptch1flox/flox and non-transgenic littermate controls at e18.5. Immunofluorescence analysis revealed the presence of all three cell types in transgenic embryos (Fig. 4A), with endocrine cells embedded in exocrine tissue. However, morphometric analysis revealed that the combined area of the three epithelial cell population was significantly smaller in transgenic embryos (Fig. 4B). Note that the reduction of epithelial area was more profound than the reduction in pancreatic weight, likely representing the contribution of mesenchyme hyperplasia to the latter (compared Figs 2D and 4B). In addition, the ratio between endocrine (combined insulin- and glucagon-positive area) and exocrine area (amylase-positive area) was smaller in transgenic embryos (Fig. 4C), implicating these two cellular compartments were differentially affected by increased mesenchymal Hh signaling.
Figure 4. Reduced pancreatic epithelium upon increased mesenchymal Hh signaling.
(A) Immunofluorescence analysis of dissected pancreatic tissues from Nkx3.2-Cre;Ptchflox/flox (right panel) and non-transgenic (left panel) stained with antibodies against insulin (‘Ins’; green), glucagon (‘Gluc’; red) and amylase (‘Amy’; blue). (B) Bar diagram (mean ± SD) shows epithelial area (combined glucagon, insulin, and amylase-positive areas) in transgenic (orange bar) and non-transgenic (cyan bar) littermates. n = 3. p value: ***p < 0.005, as compared to non-transgenic control, determined using Student’s t-test. (C) Bar diagram (mean ± SD) shows endocrine area (combined glucagon and insulin -positive areas) divided by exocrine area (amylase-positive area), in transgenic (orange bar) and non-transgenic (cyan bar) littermates. n = 3. p value: *p < 0.05, as compared to non-transgenic control, determined using Student’s t-test. (D) Immunofluorescence analysis of dissected pancreatic tissues from Nkx3.2-Cre;Ptchflox/flox (right panel) and non-transgenic (non tg; left panel) stained with an antibody against amylase (‘Amy’; red). Shown are representative fields. (E) Histological analysis of pancreatic tissue of e18.5 Nkx3.2-Cre;Ptchflox/flox transgenic (right panel) and non-transgenic (‘Non tg’; left panel) littermate embryos. Tissue-sections stained with Hematoxylin and Eosin (H&E). Shown are representative fields. (F) Bar diagram (mean ± SD) shows Amylase-positive area in transgenic (orange bar) and non-transgenic (cyan bar) littermates. n = 3. p value: ***p < 0.005, as compared to non-transgenic control, determined using Student’s t-test.
Morphology of exocrine tissue was disrupted in transgenic embryos, with more compacted cellular distribution (Fig. 4A,D and E). Our analysis indicated that the typical acellular areas normally found between adjacent lobes is lost in transgenic embryos, and is filled by mesenchymal cells (Figs 3B and 4E). As expected from the reduced epithelial area, morphometric analysis revealed smaller amylase-positive area in Nkx3.2-Cre;Ptch1flox/flox e18.5 embryos as compared to littermate control (Fig. 4F). To conclude, our findings indicate that deregulated mesenchymal Hh signaling impairs growth and morphology of the exocrine pancreas.
Abnormal islet morphology in Nkx3.2-Cre;Ptch1 flox/flox pancreas
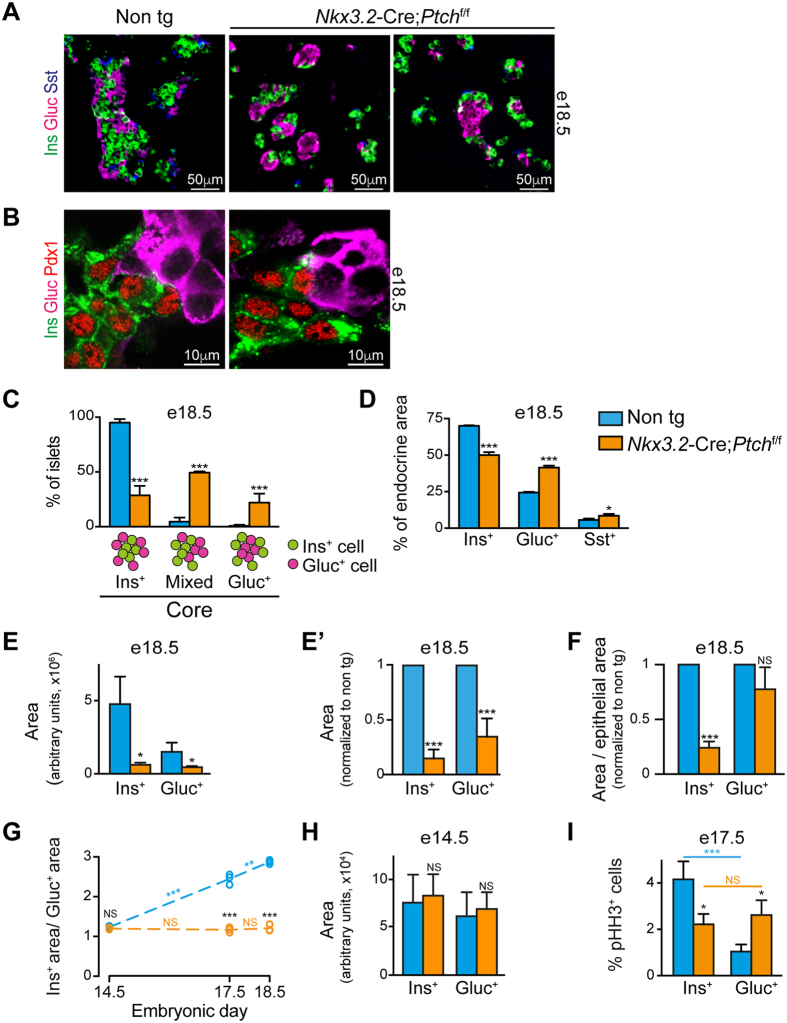
Our analysis indicates that endocrine mass is affected more so than that of exocrine from elevated Hh signaling (Fig. 4C). To analyze for potential changes in endocrine cells upon mesenchymal Ptch1 deletion, pancreatic tissues of Nkx3.2-Cre;Ptch1flox/flox and non-transgenic littermates e18.5 embryos were immune-stained for insulin, glucagon, and somatostatin. Our analysis revealed that while all three endocrine cell populations are present in transgenic embryos, islet morphology was abnormal (Fig. 5A). Of note, Pdx1 expression by insulin-positive, but not by glucagon-positive cells in transgenic pancreatic tissue pointed to appropriate cell fate acquisition36 (Fig. 5B).
Figure 5. Abnormal endocrine development upon increased mesenchymal Hh signaling.
Pancreatic tissues from Nkx3.2-Cre;Ptchflox/flox transgenic (orange) and non-transgenic (‘Non tg’; cyan) littermate embryos were analyzed at indicated embryonic days. (A,B) Immunofluorescence analysis of dissected e18.5 pancreatic tissues stained with antibodies against insulin (‘Ins’; green), glucagon (‘Gluc’; magenta) and either somatostatin (‘Sst’; blue) (A) or Pdx1 (red)(B). Shown are representative fields. (C) Bar diagram (mean ± SD) shows the percentage of islets with insulin-positive (‘Ins+ core’; left bars), glucagon-positive (‘Gluc+ core’; right bars) core, or core made of both cell types (‘Mixed’; middle bars) at e18.5. Cartoon show illustration of islets with indicated cores. n = 3. (D) Bar diagrams (mean ± SD) show the percentage of insulin (Ins+), glucagon (Gluc+) and somatostatin (Sst+) positive areas of total endocrine area (set as the combined area of all three cell types) at e18.5. n = 3. (E,E’) Bar diagram (mean ± SD) shows measurement of insulin (Ins+) and glucagon (Gluc+) positive areas at e18.5, represented in arbitrary units (E) or normalized to non-transgenic littermate control (non tg; set to 1)(E’). n = 3. (F) Bar diagram (mean ± SD) shows measurement of insulin (Ins+) and glucagon (Gluc+) positive areas at e18.5, divided by total epithelial area (as shown in Fig. 4B) and normalized to non-transgenic control (non tg; set to 1). n = 3. (G) Scattered dot plot showing the ratio between insulin (Ins+) and glucagon (Gluc+) positive areas in pancreatic tissues of e14.5, e17.5 and e18.5 embryos. Dashed lines represent trend lines between adjacent ages. (H) Bar diagram (mean ± SD) shows measurement of insulin (Ins+) and glucagon (Gluc+) positive areas at e14.5, represented in arbitrary units. n = 3. (I) Bar diagram (mean ± SD) shows the percentage of insulin (Ins+), glucagon (Gluc+) –positive cells co-expressing phosphorylated Histone H3 (pHH3). n = 3. P values: *p < 0.05, **p < 0.01, ***p < 0.005, NS = non-significant. Comparison between transgenic and non-transgenic embryos are marked with black font, while comparison between samples of the same genotype are marked with orange (transgenic embryos) and cyan (non-transgenic embryos) fonts, all determined using student’s t-test.
In mice, β-cells populate the islet core, while α- and δ-cells are found in the islet periphery, forming a mantle2. As shown in Fig. 5A, transgenic islets displayed a disrupted organization, with some having a α-cell core and β- and δ-cell mantle. To quantify the observed morphological changes, we divided islets into three groups based on their core: distinct β-cell core, distinct α-cell core, or with a core formed by both cell types (‘mixed’; Fig. 5C). A vast majority of islets in non-transgenic pancreatic tissue had the typical β-cell core, with few having a core containing both β- and α-cells (Fig. 5C). In contrast, less than one-third of islets in e18.5 Nkx3.2-Cre;Ptch1flox/flox pancreatic tissue had a defined β-cell core, with about one-half of their islets exhibited a core containing both β- and α-cells. Interestingly, while we observed no islets with a α-cell core in non-transgenic pancreatic tissues, around one-fifth of transgenic islets had a distinct α-cell core (Fig. 5C). Thus, our findings indicate that deregulated mesenchymal Hh signaling leads to disrupted islet morphology.
Abnormal endocrine composition and mass upon deletion of mesenchymal Ptch1
To analyze if the observed abnormal islet morphology is associated with changes in islet cellular composition, we directly compare the portion of endocrine cell types. To this end, we measured insulin+, glucagon+ and somatostatin+ area in pancreatic tissues of Nkx3.2-Cre;Ptch1flox/flox transgenic and control e18.5 embryos. As shown in Fig. 5D, non-transgenic tissues exhibit the expected endocrine cell ratio2, with ~70% being insulin+, ~24% being glucagon+ and the remaining ~6% being somatostatin+. However, transgenic pancreatic tissues had a reduced insulin+ portion to ~50% of endocrine cells, whereas the portion of glucagon+ increased to ~41% and that of somatostatin+ to ~9% of endocrine cells (Fig. 5D). To conclude, our findings indicate that regulated mesenchymal Hh signaling is required to maintain the stereotypical islet cellular composition.
To directly analyze for potential changes in α- and β-cell mass upon deregulation of mesenchymal Hh signaling, we analyzed their area. Morphometric analysis revealed a significantly reduced area in both these cell populations in e18.5 Nkx3.2-Cre;Ptch1flox/flox embryos, as compared to littermate control (Fig. 5E and E’). Furthermore, while the reduction of glucagon+ area was proportional to the reduced epithelial area in transgenic embryos, the reduction in insulin+ area was more profound than the reduction in total epithelial area (Fig. 5F). In agreement with other studies23,24,25, our analysis indicates that β-cell mass is affected more so than that of α-cells from elevated Hh signaling.
Regulated mesenchymal Hh signaling is required for endocrine cell-specific proliferation rates
Our analysis implicates that manipulating Hh signaling in the pancreatic mesenchyme differentially affected α- and β- cell development. To directly test this possibility, we calculated the ratio between insulin+ and glucagon+ area at e14.5, e17.5, and e18.5 in Nkx3.2-Cre; Ptch1flox/flox embryos and littermate controls. As shown in Fig. 5G, in control embryos, insulin+ area was slightly larger (by ~1.2 fold) than glucagon+ area at e14.5, likely representing increased frequency of precursor differentiation toward a β-cell fate13, combined with larger β-cell size (Table 1)37. However, insulin/glucagon ratio significantly increased with age in non-transgenic embryos; while insulin+ area was 2.4-fold bigger than glucagon+ area at e17.5, this difference grew to 2.9-fold at e18.5 (Fig. 5G). This analysis indicates that β-cell population grows at a higher rate than α-cell population during normal pancreas development.
Table 1. Estimated β- and α- cells area at e14.5.
| Ins+ cell area (μm2) | Gluc+ cell area (μm2) | Ins+ cell area/Gluc+ cell area | |
|---|---|---|---|
| Non transgenic | 52 ± 14 | 46 ± 12 | 1.13(*) |
| Nkx3.2-Cre;Ptchf/f | 48 ± 14 | 42 ± 11 | 1.15(*) |
Cell area (Mean ± SD) was measured in non-transgenic and Nkx3.2-Cre;Ptchf/f littermate e14.5 embryos. n = 50 cells. (*) p value: p < 0.05, between β-cell and α-cell area within mouse group, determined using Student’s t-test. No significance differences were detected between cells area in non-transgenic and transgenic embryos. Ins = insulin; Gluc = glucagon.
In e14.5 Nkx3.2-Cre;Ptch1flox/flox embryos, insulin/glucagon ratio was comparable to that of control (ratio of 1.2) (Fig. 5G). Of note, transgenic embryos displayed comparable insulin+ and glucagon+ area to their littermate controls at this age (Fig. 5H). Interestingly, this ratio remained constant in transgenic embryos (ratio of ~1.2) at the three analyzed ages (Fig. 5G), implicating similar growth rates of the two cell populations upon deregulated mesenchymal Hh signaling.
Our analysis indicates that β-cell population grows at a higher rate than the α-cell population during normal development, but not upon increased mesenchymal Hh signaling (Fig. 5G). As this is observed also between e17.5 and e18.5, after differentiation of the two populations from common endocrine precursors had ceased1, we analyzed for potential differences in cell proliferation at e17.5. To this end, we analyzed the portion of α- and β- cells expressing the proliferation marker phosphorylated Histone H3 (pHH3) in Nkx3.2-Cre;Ptch1flox/flox and non-transgenic e17.5 embryos. As shown in Fig. 5I, the portion of pHH3+ β-cells in non-transgenic embryos was significantly higher (~4-fold) than that of α-cells. In contrast, the portions of pHH3+ β-cells and α-cells in transgenic embryos were comparable (Fig. 5I). Of note, while the rate of β-cell proliferation at e17.5 decreased in transgenic embryos as compared to control, the rate of α-cell proliferation increased.
This set of experiments indicate that during normal pancreas development, β-cells grow at a higher rate than α-cells to establish proper islet cellular composition. Furthermore, our results suggest that this cell specific growth rates require regulated Hh signaling in mesenchymal cells.
Deregulated Hh signaling leads to elevated mesenchymal mass
Mesenchymal cells surround islet of Langerhans, and their depletion abrogated endocrine cell growth11. To analyze whether endocrine-associated mesenchymal cells are affected by increased Hh signaling, pancreatic tissues of Nkx3.2-Cre;R26R-EYFP;Ptch1flox/flox and Nkx3.2-Cre;R26R-EYFP;Ptch1flox/+ littermate control e18.5 embryos were immuno-stained for YFP, insulin, and glucagon. As shown in Fig. 6A, while mesenchymal cells formed a thin, cell-wide layer surrounding the islet of Langerhans in control embryos11, this layer was considerably thickened in Nkx3.2-Cre;R26R-EYFP;Ptch1flox/flox embryos (Fig. 6A), similarly to the observed thickening of this layer in the exocrine tissue (Fig. 3).
Figure 6. Deregulated Hh signaling leads to mesenchymal hyperplasia.
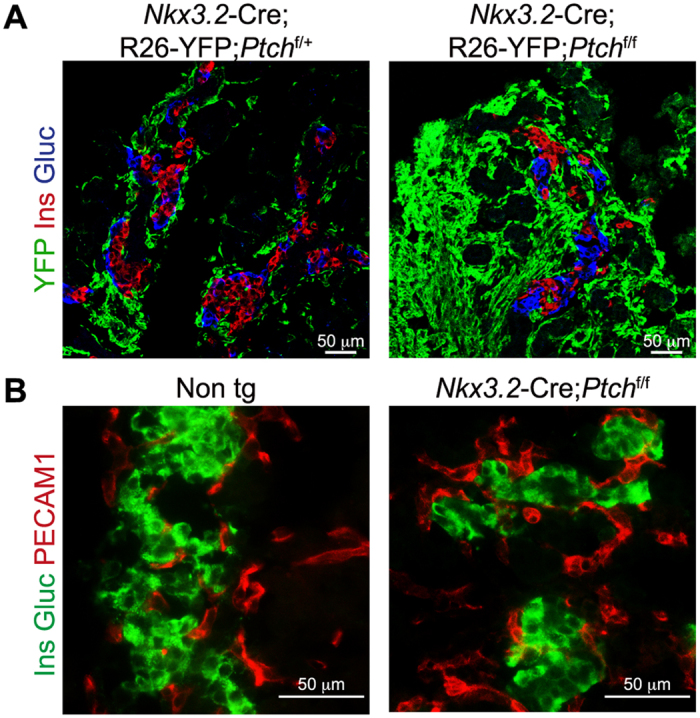
(A) Immunofluorescence analysis of dissected pancreatic tissues from Nkx3.2-Cre;R26-YFP;Ptchflox/flox (right) and Nkx3.2-Cre;R26-YFP;Ptchflox/+ (left) control littermate e18.5 embryos. Tissues were stained with antibodies against YFP (green), insulin (‘Ins’; red) and glucagon (‘Gluc’; blue). Shown are representative fields. (B) Immunofluorescence analysis of dissected pancreatic tissues from Nkx3.2-Cre; Ptchflox/flox transgenic (right) and non-transgenic (Non tg; left) stained with antibodies against insulin (‘Ins’; green), glucagon (‘Gluc’; green) and the endothelial marker PECAM1 (red). Shown are representative fields.
Endothelial cells were shown to regulate β-cell development38,39. Although endothelial cells are not targeted by the Nkx3.2-Cre mouse line11,40, they might be affected by changes in the pancreatic mesenchyme. We therefore stained pancreatic tissues of Nkx3.2-Cre;Ptch1flox/flox and non-transgenic e18.5 embryos for the endothelial marker Platelet Endothelial Cell Adhesion Molecule 1 (PECAM1). Our analysis indicated the presence of endothelial cells in and around islets of both transgenic and control embryos, with similar distribution (Fig. 6B). To conclude, our findings point to increased mesenchymal mass, without apparent change in endothelial area, around islet of Langerhans upon deregulated Hh signaling.
Discussion
Regulation of Hh signaling is essential for proper pancreas development. Here, we analyzed the contribution of mesenchymal Hh signaling to this process. Our results indicate that the ability of the pancreatic mesenchyme to support organogenesis depends on proper regulation of Hh signaling in these cells. To increase Hh signaling in the pancreatic mesenchyme, we generated Nkx3.2-Cre;Ptch1flox/flox embryos, in which two copies of Ptch1 were deleted in this tissue. Deregulated Hh signaling in mesenchymal cells was sufficient to disrupt epithelial growth, affecting both the endocrine and the exocrine pancreas. However, mesenchymal growth was increased, leading to hyperplasia of this cell layer. We further observed disrupted endocrine cellular composition, with a reduced β-cell portion and abnormal islet morphology. Thus, our findings indicate that the cell-specific growth rates of epithelial cell populations depend on the pancreatic mesenchyme, and requires regulated Hh signaling activity in this cell layer. To conclude, we showed that mesenchymal Hh signaling is required for pancreatic growth and establishment of its cellular composition.
Islets of Langerhans display a characteristic cellular composition, determined during development2,13. Our results indicate that in the mouse embryo, pancreatic endocrine cells exhibit specific growth rates, with the β-cell population growing at a higher rate than the α-cell population. In part, this could be an outcome of a higher tendency of endocrine precursors to differentiate to β-cells than to alternative cell fates13. In addition, our results suggest that cell-specific proliferation rate might contribute to the stereotypical islet composition, when β-cells proliferate at a higher rate than α-cells do. Deregulated Hh signaling in pancreatic mesenchymal cells, achieved by deletion of Ptch1 in these cells, led to similar β- and α- cell growth rates toward end of gestation, likely contributing to the observed abnormal islet composition. While we observed abnormal cell proliferation rates in transgenic embryos, this could not fully explain the dramatic reduction in β- and α-cell mass. It is therefore possible that endocrine cells proliferate at a higher rate at earlier developmental stages. Alternatively, although normal β- and α- mass was observed at e14.5, their differentiation rate was affected by deregulated mesenchymal Hh signaling. Of note, β-cell development was shown by others to be more affected than α-cells from deregulated pancreatic Hh25, further suggesting their specific growth rate is dependent on restrained Hh signaling. While β-cell function was shown to require cells in the islet microenvironment38,40, the postnatal lethality of Nkx3.2-Cre;Ptch1flox/flox mice prevents us from being able to directly study the role of mesenchymal Hh signaling in this process. Nevertheless, the abnormal endocrine composition observed in transgenic mice would have likely affected the levels of secreted hormones.
Mouse islets have a distinct β-cell core, whereas the core of human islets were reported to be populated by both α- and β-cells2,41. Recent studies suggest that the morphology of human islets depends on their size, whereas small islets resemble the morphology of mouse islets, with a distinct β-cell core42,43. Furthermore, the arrangement of endocrine cells within the islets allows for proper homotypic and heterotypic interactions, essential for proper functioning2,44. We observed that increased mesenchymal Hh signaling leads to abnormal islet organization, with most islets lacking the typical β-cell core. It could therefore be possible that the α- to β- cell ratio dictates islet morphology, and that the abnormal endocrine cell ratio in transgenic embryos leads to the abnormal islet morphology. This possibility is supported by the abnormal islet morphology observed upon increased β-cell death during development45. Alternatively, similarly to neurons46, mesenchymal cells may directly dictate pancreatic endocrine cell arrangement and islet morphology.
Pancreas morphogenesis and growth depend on proper interactions of the developing epithelium with cells in its surrounding. Mesenchymal-epithelial interaction was shown to be promote epithelial branching11,47. Our findings indicate that deregulated mesenchymal Hh signaling leads to hyperplasia of mesenchymal cells and abrogated epithelial expansion and branching. The pancreatic endothelium was shown to restrain exocrine growth, when hyper-vascularization repress pancreas expansion and branching48,49,50. It is therefore possible that, similarly to endothelial cells, mesenchymal hyperplasia does not allow proper mesenchymal-epithelial interactions, leading to the observed morphological phenotype. Alternatively, yet to be identified Hh-dependent mesenchymal cues may regulate pancreatic growth and branching.
The role of Hh signaling in pancreas development was established by a series of studies that manipulated this pathway in both epithelial and mesenchymal compartments19,21,23,24,25. These studies showed that restrained pancreatic Hh signaling is crucial for epithelial expansion and proper β-cell mass and function. Epithelial and β-cell specific manipulations of this pathway recapitulated some of these phenotypes, including reduced endocrine mass and impaired β-cell function, indicating a cell intrinsic role of Hh signaling22,26,27. By manipulating this pathway in the pancreatic mesenchyme, but not in its epithelium, we directly showed a cell extrinsic roles of Hh signaling in epithelial expansion. Of note, we could not observe ectopic pancreas in Nkx3.2-Cre;Ptch1f/f embryos, further indicating that this phenomena is an outcome of deregulated Hh signaling in the pancreatic epithelium51. Importantly, neither epithelial nor mesenchymal -specific elevation of Hh signaling fully recapitulate the pancreatic agenesis phenotype observed upon pancreas-wide manipulation of this pathway19,23,24,25. The different manipulations of this pathway (removal of regulatory elements, ectopic ligand, and transcription factors’ expression) may lead to different levels of pathway activation. Alternatively, deregulating Hh signaling in both the epithelium and the mesenchyme might have a synergetic, negative effect on epithelial growth. The severity of pancreatic phenotype observed upon systemic manipulation of Ptch1 expression24, as compared to the phenotype described here upon mesenchymal manipulation of this gene, supports the requirement of regulated Hh signaling in both pancreatic epithelium and mesenchyme.
Hh signaling was shown to be required for proliferation of mesenchymal cells of the gastrointestinal tract32. While along the gut tube mesenchymal cells form the smooth muscle layer that controls its local movement, the adult pancreas lacks this layer and contains relatively few mesenchymal cells (including pancreatic stellate cells, vSMCs, and pericytes)35,52. Therefore, the expression of Hh ligands along the gut tube, and their exclusion from the developing pancreas, may reflect a differential need for mesenchymal expansion19,20,32. This notion was first suggested by Apelqvist and colleagues in 1997, in a seminal study reporting acquisition of a gut-like phenotype by pancreatic mesenchymal cells upon ectopic Shh expression19, and was further supported by others23,24,25. Furthermore, Hh signaling was shown to promote stroma expansion during the progression of pancreatic ductal adenocarcinoma (PDAC)53. Here, we were able to directly show that elevated Hh signaling leads to expansion of the mesenchymal layer in a cell-autonomous manner. Hence, regulated Hh signaling may be required for establishing a proper epithelial-mesenchymal ratio in the digestive system, allowing for proper size and functioning of these organs.
Materials and Methods
Mice
All experiments were performed according to protocols approved by the Committee on Animal Research at Tel Aviv University. Nkx3.2 (Bapx1)-Cre (Nkx3–2tm1(cre)Wez)29, Ptch1LacZ (Ptch1tm1Mps/J)30, Ptch1flox (Ptch1tm1Bjw)28, R26-YFP (Gt(ROSA)26Sortm1(EYFP)Cos/J)34 mouse lines were used in this study. Noon on the day a vaginal plug was detected was considered as embryonic day 0.5.
X-gal staining
Dissected gastrointestinal tissues were fixed with Paraformaldehyde (4%) for 2 h and incubated overnight at room temperature with X-gal (5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside; 2.5 mg/ml; Sigma) diluted in PBS containing Potassium Ferrocyanide (4.35 mM), Potassium Ferricyanide (5 mM), NP-40 (0.02%), MgCl2 (2 mM), followed by a second round of fixation in Paraformaldehyde (4%) overnight. Tissues were then embedded in paraffin, sectioned, and counter-stained with nuclear Fast Red (Vector). Images were acquired using Keyence BZ-9000 microscope (Biorevo).
Hematoxylin and Eosin staining
Dissected pancreatic tissues were fixed with Paraformaldehyde (4%) for 2 hours, embedded in paraffin wax and sectioned. Following deparaffinization, tissue sections were stained with Meyer’s Hematoxylin (Sigma) followed by staining with Eosin (Sigma). Images were acquired using Keyence BZ-9000 microscope (Biorevo).
Immunofluorescence
Dissected pancreatic tissues were fixed with Paraformaldehyde (4%) for 2–4 hours. Tissue were embedded in paraffin wax, sectioned to 5 μm sections and stained using the following primary antibodies: rabbit anti-Amylase (Sigma, Catalog #A8273), rabbit anti-Glucagon (Millipore, Catalog #AB932), mouse anti-Glucagon (Sigma, Catalog #G2654), guinea pig anti-Insulin (DAKO, Catalog #A0564), rabbit anti-phosphorylated Histone H3 (Millipore, Catalog #06–570), rat anti-Somatostatin (Millipore, Catalog #MAB354). Alternatively, following their fixation, tissues were embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek), cryo-sectioned to 11 μm sections and stained using the following primary antibodies: mouse anti-Desmin (Dako; Catalog #M0760), rabbit anti-Glucagon (Millipore, Catalog #AB932), mouse anti-Glucagon (Sigma, Catalog #G2654), guinea pig anti-Insulin (DAKO, A0564), rabbit anti-Pdx1 (Millipore, Catalog #MM07696), rat anti-PECAM1 (BD, Catalog #553370), anti-αSMA (Abcam, Catalog #Ab5694) and chicken anti-YFP/GFP (Abcam, Catalog #Ab13970). Staining was followed by staining with AlexaFluor tagged secondary antibodies (1:500, Invitrogen) and mounting with DAPI-containing Vectashield media (Vector). Images were acquired using Keyence BZ-9000 microscope (Biorevo) and SP8 confocal microscope (Leica).
Morphometric quantifications
For all measurements presented in this study the following regimen was applied: the entire pancreatic tissue, including both dorsal and ventral buds, was embedded in paraffin wax and cut into 5 μm thick sections. For e18.5 and e17.5 embryos, every fifth section (20% of total tissue) was immuno-stained with indicated antibodies (as described above), where each transgenic tissue was processed and stained in parallel with its littermate control. For e14.5 embryos, half of the sections was immuno-stained with indicated antibodies (as described above), where each transgenic tissue was processed and stained in parallel with its littermate control. Sections were automatically imaged using Keyence BZ-9000 microscope (Biorevo). For all quantifications, with the exception of the measurement of islet morphology and pHH3 expression, all acquired images were analyzed using imageJ software (NIH). For analysis of islet morphology, 50–70 islets from each embryo were manually scored, blind to genotype. For analysis of percentage of pHH3 expressing cells, at least 150 cells from each analyzed cell type in each embryo were manually scored, blind to genotype.
Quantitative PCR
RNA was extracted from isolated tissues using PureLink RNA Micro Kit (Invitrogen), followed by a reverse transcription reaction with SuperScript VILO (Invitrogen). Gli1 expression levels were detected with Taqman assays (Invitrogen) and was normalized to Cyclophilin (Primers: GGCCGATGACGAGCCC, TGTCTTTGGAACTTTGTCTGCAA, Probe: TGGGCCGCGTCTCCTTCGA), using StepOne Real-Time PCR System (Thermo Fisher).
Statistics
p-Values were determined using unpaired, two-tailed student’s t test.
Additional Information
How to cite this article: Hibsher, D. et al. Pancreatic Mesenchyme Regulates Islet Cellular Composition in a Patched/Hedgehog-Dependent Manner. Sci. Rep. 6, 38008; doi: 10.1038/srep38008 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We thank Daria Baer, Eleonor Rachi and Gayathri Ramakrishnan for technical assistance, Helen C. Guez for critical reading of the manuscript, and members of the Landsman laboratory for helpful discussion. This work was supported by a Marie-Curie Career Integration Grant (FP7-CIG-333800)(to L.L.). This work was carried out in partial fulfillment of the requirements for a Ph.D. degree for D.H. from the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Footnotes
Author Contributions D.H. preformed experiments and analyzed data, A.E. preformed experiments, N.O. analyzed data, and L.L. designed the study, supervised the project and wrote the manuscript.
References
- Gu G., Dubauskaite J. & Melton D. A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002). [DOI] [PubMed] [Google Scholar]
- Cabrera O. et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103, 2334–2339 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes G. K. Developmental biology of the pancreas: a comprehensive review. Dev Biol 326, 4–35 (2009). [DOI] [PubMed] [Google Scholar]
- Duvillie B., Stetsyuk V., Filhoulaud G., Guillemain G. & Scharfmann R. Control of pancreatic development by intercellular signals. Biochem. Soc. Trans 36, 276 (2008). [DOI] [PubMed] [Google Scholar]
- Miyatsuka T., Kosaka Y., Kim H. & German M. S. Neurogenin3 inhibits proliferation in endocrine progenitors by inducing Cdkn1a. Proc Natl Acad Sci USA 108, 185–190 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis E. D., Bechard M. E. & Wright C. V. E. Feedback control of growth, differentiation, and morphogenesis of pancreatic endocrine progenitors in an epithelial plexus niche. Genes Dev 29, 2203–2216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan A. et al. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 128, 5109–5117 (2001). [DOI] [PubMed] [Google Scholar]
- Ahnfelt-Rønne J., Ravassard P., Pardanaud-Glavieux C., Scharfmann R. & Serup P. Mesenchymal bone morphogenetic protein signaling is required for normal pancreas development. Diabetes 59, 1948–1956 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golosow N. & Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol 4, 242–255 (1962). [DOI] [PubMed] [Google Scholar]
- Elghazi L., Cras-Méneur C., Czernichow P. & Scharfmann R. Role for FGFR2IIIb-mediated signals in controlling pancreatic endocrine progenitor cell proliferation. Proc Natl Acad Sci USA 99, 3884–3889 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L. et al. Pancreatic mesenchyme regulates epithelial organogenesis throughout development. Plos Biol 9, e1001143 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B. M., Hrycaj S. M., Newman M., Li Y. & Wellik D. M. Mesenchymal Hox6 function is required for mouse pancreatic endocrine cell differentiation. Development 142, 3859–3868 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgraz R. & Herrera P. L. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development 136, 3567–3574 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 13, 103–114 (2007). [DOI] [PubMed] [Google Scholar]
- van den Brink G. R. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiological Reviews 87, 1343–1375 (2007). [DOI] [PubMed] [Google Scholar]
- Lau J., Kawahira H. & Hebrok M. Hedgehog signaling in pancreas development and disease. Cell Mol Life Sci 63, 642–652 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham P. W. & McMahon A. P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15, 3059–3087 (2001). [DOI] [PubMed] [Google Scholar]
- Pak E. & Segal R. A. Hedgehog Signal Transduction: Key Players, Oncogenic Drivers, and Cancer Therapy. Dev Cell 38, 333–344 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelqvist A., Ahlgren U. & Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol 7, 801–804 (1997). [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M., Melton D. A. & McMahon A. P. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127, 2763–2772 (2000). [DOI] [PubMed] [Google Scholar]
- Hebrok M., Kim S. K., St Jacques B., McMahon A. P. & Melton D. A. Regulation of pancreas development by hedgehog signaling. Development 127, 4905–4913 (2000). [DOI] [PubMed] [Google Scholar]
- Lau J. & Hebrok M. Hedgehog signaling in pancreas epithelium regulates embryonic organ formation and adult beta-cell function. Diabetes 59, 1211–1221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahira H. et al. Combined activities of hedgehog signaling inhibitors regulate pancreas development. Development 130, 4871–4879 (2003). [DOI] [PubMed] [Google Scholar]
- Nakayama S. et al. Dose-dependent requirement of patched homologue 1 in mouse pancreatic beta cell mass. Diabetologia 51, 1883–1892 (2008). [DOI] [PubMed] [Google Scholar]
- Kawahira H., Scheel D. W., Smith S. B., German M. S. & Hebrok M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol 280, 111–121 (2005). [DOI] [PubMed] [Google Scholar]
- Cervantes S., Lau J., Cano D. A., Borromeo-Austin C. & Hebrok M. Primary cilia regulate Gli/Hedgehog activation in pancreas. Proc Natl Acad Sci USA 107, 10109–10114 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L., Parent A. & Hebrok M. Elevated Hedgehog/Gli signaling causes beta-cell dedifferentiation in mice. Proc Natl Acad Sci USA 108, 17010–17015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T. et al. Patched 1 conditional null allele in mice. Genesis 36, 158–161 (2003). [DOI] [PubMed] [Google Scholar]
- Verzi M. P. et al. Role of the homeodomain transcription factor Bapx1 in mouse distal stomach development. Gastroenterology 136, 1701–1710 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich L. V., Milenkovic L., Higgins K. M. & Scott M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997). [DOI] [PubMed] [Google Scholar]
- Tribioli C. & Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development 126, 5699–5711 (1999). [DOI] [PubMed] [Google Scholar]
- Mao J., Kim B.-M., Rajurkar M., Shivdasani R. A. & McMahon A. P. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 137, 1721–1729 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecksher-Sorensen J. et al. The splanchnic mesodermal plate directs spleen and pancreatic laterality, and is regulated by Bapx1/Nkx3.2. Development 131, 4665–4675 (2004). [DOI] [PubMed] [Google Scholar]
- Srinivas S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1, 4 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards O. C., Raines S. M. & Attie A. D. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev 31, 343–363 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren U., Jonsson J. & Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 12, 1763–1768 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimny M. L. & Blackard W. G. The Surface structure of isolated pancreatic islet cells. Cell Tissue Res. 164, 467–471 (1975). [DOI] [PubMed] [Google Scholar]
- Nikolova G. et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell 10, 397–405 (2006). [DOI] [PubMed] [Google Scholar]
- Reinert R. B. et al. Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes 62, 4154–4164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson A. et al. Islet pericytes are required for beta-cell maturity. Diabetes 65, 3008–3014 (2016). [DOI] [PubMed] [Google Scholar]
- Brissova M. et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53, 1087–1097 (2005). [DOI] [PubMed] [Google Scholar]
- Bosco D. et al. Unique Arrangement of α- and β-Cells in Human Islets of Langerhans. Diabetes 59, 1202–1210 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S., Sullivan B. A. & Weir G. C. Human Islet Morphology Revisited: Human and Rodent Islets Are Not So Different After All. J. Histochem. Cytochem. 63, 604–612 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco D., Orci L. & Meda P. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp Cell Res 184, 72–80 (1989). [DOI] [PubMed] [Google Scholar]
- Tornovsky-Babeay S. et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in β cells. 19, 109–121 (2014). [DOI] [PubMed]
- Borden P., Houtz J., Leach S. D. & Kuruvilla R. Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep 4, 287–301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan G. et al. Cdc42-mediated tubulogenesis controls cell specification. Cell 139, 791–801 (2009). [DOI] [PubMed] [Google Scholar]
- Pierreux C. E. et al. Epithelial: Endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev Biol 347, 216–227 (2010). [DOI] [PubMed] [Google Scholar]
- Magenheim J. et al. Blood vessels restrain pancreas branching, differentiation and growth. Development 138, 4743–4752 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand F. W. et al. Growth-limiting role of endothelial cells in endoderm development. Dev Biol 352, 267–277 (2011). [DOI] [PubMed] [Google Scholar]
- Xuan S. & Sussel L. GATA4 and GATA6 regulate pancreatic endoderm identity through inhibition of hedgehog signaling. Development 143, 780–786 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkan M. et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 61, 172–178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]