Abstract
The vertebrate pancreas is comprised of a highly branched tubular epithelium, which is intimately associated with an extensive and specialized vasculature. While we know a great deal about basic vascular anatomy of the adult pancreas, as well as islet capillaries, surprisingly little is known about the ontogeny of its blood vessels. Here, we analyze development of the pancreatic vasculature in the mouse embryo. We show that pancreatic epithelial branches intercalate with the fine capillary plexus of the surrounding pancreatic mesenchyme. Endothelial cells (ECs) within this mesenchyme are heterogeneous from the onset of organogenesis. Pancreatic arteries take shape before veins, in a manner analogous to early embryonic vessels. The main central artery forms during mid-gestation, as a result of vessel coalescence and remodeling of a vascular plexus. In addition, we show that vessels in the forming pancreas display a predictable architecture that is dependent on VEGF signaling. Over-expression of VEGF disrupts vascular patterning and arteriovenous differentiation within the developing pancreas. This study constitutes a first-time cellular and molecular characterization of pancreatic blood vessels, as they coordinately grow along with the pancreatic epithelium.
Keywords: pancreas, epithelium, endothelial, blood vessel, VEGF, artery, vein, plexus, heterogeneity
INTRODUCTION
Blood vessel formation is essential to embryonic development and adult tissue function (see review see(Herbert and Stainier, 2011). During early embryogenesis, endothelial cell (EC) precursors called angioblasts arise in the mesoderm and arrange into cords. They then take on a squamous cell morphology, open lumens and give rise to vascular tubes. This initial de novo development of vessels from angioblasts is called vasculogenesis. Subsequently, emerging vessels change in various ways by processes collectively termed vascular remodeling. New vessels can develop via sprouting angiogenesis, where nascent sprouts branch from a preexisting vessel. An existing vessel can also split into multiple vessels via intussusceptive angiogenesis. Conversely, smaller vessels can coalesce and fuse into larger ones. As these remodeling processes take place, arteries and veins differentiate under the influence of both genetic and hemodynamic mechanisms. The resulting mature vasculature is thus a hierarchical tree made up of arteries, arterioles, capillaries, venules and veins, each with different properties that enable vessels to serve specific functions. Arteries and veins have been shown to be established relatively early in the embryo, although they exhibit some plasticity for a time. Additionally, tissues develop distinct organ-specific capillary beds that carry out organ-specific functions. Therefore, the embryonic endothelium has to differentiate heterogeneously to form regionally adapted vessels throughout the body.
Once organ primordia emerge in the early embryo, their growth must be coordinated with vascular development. Indeed, blood vessels align with extending epithelial branches in branching organs, such as lungs and salivary glands (Lu and Werb, 2008). With respect to their ontogeny, blood vessels in many organs were long believed to arise as a result of angiogenesis, however peripheral vasculogenesis has been shown to contribute significantly to organ-specific capillary beds (Drake and Fleming, 2000). In fact, a few studies have suggested that organ-associated vascular beds arise via a combination of vasculogenesis and angiogenesis to build functional organs (Sequeira Lopez and Gomez, 2011). However, despite such studies, much remains to be understood about patterning of blood vessels and establishment of transcriptional heterogeneity during the building of organ-specific vasculatures.
Recent efforts have been directed to elucidate functional relationships between epithelia and endothelium in organs and tissues. Understanding this relationship is of great value to the fields of vascular development and tissue stem cells. In the pancreas, such a functional relationship is required for organ development. The dorsal pancreatic bud evaginates at embryonic day 9.5 (E9.5) from the dorsal gut endoderm towards the aorta, driven by epithelial stratification. At this time (E10.5), the bud consists of undifferentiated and largely apolar progenitor cells. As the epithelium remodels and branches, it undergoes de-stratification and cells regain apico-basal polarity (E10.5-E11.5). During these early stages (E9.5-12.0), termed the primary transition, very little cellular differentiation occurs. Around E12.0, the secondary transition begins, with rapid growth and branching of the epithelium coinciding with widespread cellular differentiation. Studies have shown that ablation of blood vessels associated with the pancreatic bud leads to failure in pancreas development: Removal of the aorta prior to bud initiation, or blocking of vascular growth through VEGFR2 inhibition after budding, results in blockade of beta cell differentiation, or abnormal epithelial growth and differentiation, respectively (Lammert et al., 2001; Magenheim et al., 2011b; Pierreux et al., 2010; Sand et al., 2011b). Conversely, Pdx1-driven VEGF over-expression (Pdx1-tet-VEGF) and hypervascularization lead to pancreatic growth arrest, failed cellular differentiation and islet disruption (Cai et al., 2012; Magenheim et al., 2011b). Thus, pancreatic vasculature profoundly impacts development of the pancreatic progenitor epithelium.
Despite the well-established role for developing vasculature in pancreatic morphogenesis and differentiation, how the pancreatic vasculature develops is poorly understood. While vascular development has been characterized in several organs, a comprehensive analysis of pancreatic vascular development has been missing (Coveney et al., 2008; Lazarus et al., 2011; Robert et al., 1998). Furthermore, it has become increasingly clear that blood vessels play different roles over the course of pancreatic development (see review(Villasenor and Cleaver, 2012). Thus, a better understanding of pancreatic vascular development is needed to dissect the role of the vasculature during pancreatic growth and differentiation. Here, we provide an in-depth characterization of pancreatic vascular development. We explore when and how the pancreatic epithelium becomes vascularized, as well as how its vessels undergo arteriovenous differentiation. We show that the initially avascular pancreatic epithelium becomes integrated with blood vessels upon branching, combined with peripheral vasculogenesis. Our data suggest that the first central vessel of the pancreas, its main artery, is formed via coalescence and remodeling of capillaries. This occurs coordinately with recruitment of smooth muscle progenitors (or mural cells). We further demonstrate that pancreatic arteries and veins emerge at predictable and apposed locations within the bud. Additionally, we identify spatially distinct and molecularly heterogeneous capillary beds in the developing pancreas. Finally, we show that excessive epithelial VEGF in the Pdx1-tet-VEGF mouse model leads to patterning failure of the pancreatic vasculature, with defects varying from ectopic or expanded to non-hierarchical or coalesced networks depending on the vascular bed.
MATERIALS AND METHODS
Mice and tissue handling
Animal husbandry was performed in accordance with protocols approved by the UT Southwestern Medical Center IACUC. CD1, EphB4-lacZ, ephrinB2-lacZ, Flk1-lacZ, Flk1-GFP and Pdx1-tet-VEGF (Magenheim et al., 2011b) mice were used for experiments herein.
E9.5-E14.5 embryos were collected from pregnant mice. The tissues were dissected in ice-cold PBS buffer. Gut tubes or stomachs attached to pancreata were isolated and fixed in 4% paraformaldehyde (PFA) in PBS with gentle rocking as follows: at 4°C overnight (o/n) for section or whole mount in situ hybridization, for 3 hours at RT for section immunostaining, or for 1 hour at RT for whole mount immunostaining. Tissues were washed three times in PBS for 5 minutes (min) each, and dehydrated to 70 % Ethanol. Tissues were stored in 70% ethanol at −20°C. Postnatal tissues were collected and fixed in a similar manner.
Sectioning
For paraffin sectioning, tissues were fixed and dehydrated as described above. Then, the tissues were rinsed twice in 100% ethanol for 5 min at room temperature (RT), twice in xylene for 30 min at RT, then a mixture of 1:1 paraplast:xylene at 60°C for 10 min, then a series of 100% paraplast at 60°C (McCormick Scientific). The tissues were then embedded in paraplast and sectioned at 10 μm with a Biocut 2030 microtome. SuperfrostPlus glass slides (Fisher) were used.
For cryosectioning, tissues were fixed as described above. Then, the tissues were rinsed in PBS three times for 5 min each and incubated in 30 % sucrose o/n at 4°C for cryoprotection. Next day, the tissues were rinsed in OCT twice for 30 min each at RT. The tissues were embedded in OCT, snap-frozen on dry ice and sectioned at 10 μm using a Leica CM-3050S cryostat. SuperfrostPlus glass slides (Fisher) were used.
Immunostaining on sections
For paraffin sections, the sections were de-paraffinized with xylene washes twice for 5 min each. Sections were rehydrated in the following Ethanol series for 1 min each: 100% (×2), 95%, 90%, 80%, 70% and 40%. Slides were washed twice in PBS for 3 min each. Sections were treated with R-Buffer A or R-Buffer B in a 2100 Retriever (Electron Microscopy Sciences). Then, sections were permeabilized in PBS+0.1%TritonX for 30 min. After a quick dip in PBS, sections were blocked in CAS-Block (Invitrogen) for 2–3 hours. Slides were incubated with primary antibody in CAS-Block o/n at 4°C. Next day, slides were washed in PBS three times 10 min each, incubated with secondary antibody for 2 hours at RT, and washed again in PBS three times 10 min each. For nuclear staining (when needed), slides were incubated in DAPI in PBS for 10 min at RT. Slides were washed in PBS three times 10 min each and mounted in Prolong Gold with or without DAPI (as needed).
For cryosections, slides were baked for 10 minutes at 55°C and rinsed in PBS three times for 10 min each. Antigen retrieval was carried out using Electron Microscopy Sciences Buffer B in retriever (as above). Sections were blocked in 5 % Normal Donkey Serum in PBS for 1 hour at RT in a humidified chamber (with PBS). Slides were incubated with primary antibody in blocking serum o/n at 4°C. Next day, slides were washed in PBS three times 10 min each, incubated in secondary antibody in blocking serum for 2 hours at RT, and washed in PBS three times for 10 min each. Nuclear staining and mounting was performed the same way as for paraffin sectioning.
For DAB immunostaining on sections, paraffin sections were de-paraffinized and rinsed in PBS as described above. Then, slides were incubated in 3 % H2O2 in Methanol to quench endogenous peroxidase activity, and washed in PBS twice for 5 min each. Antigen retrieval and blocking were carried out as described for paraffin sections. Slides were incubated with primary antibody o/n at 4°C (mouse α E-cadherin from BD Transduction, rabbit α VEGF from Abcam). Next day, slides were rinsed in PBS and incubated in secondary antibody at 1:500 for 2 hours at RT (α mouse IgG-HRP, α rabbit IgG-HRP). Slides were then washed in PBS three times for 30 min each. For color reaction, slides were incubated in DAB staining solution (DAB Substrate kit from Vectorlabs: 2 drops stock buffer, 4 drops DAB, 2drops H2O2, 2 drops Nickel in 5ml water) until staining develops as desired (2–10 min). Slides were then dehydrated in Ethanol series followed by xylene, and mounted with Permount (Fisher).
Whole mount immunostaining
Following fixation, tissues were washed in PBS three times 5 min each and dehydrated through a 25% Methanol wash for 5 min into 50% Methanol. Tissues were incubated in 50% Methanol for 1 hour at RT with gentle rocking. Tissues were dehydrated back into PBS through 5 min washes in 25% Methanol and three times in PBS. Permeabilization was carried out in PBS+1% TritonX for 3 hours at RT. Next, tissues were blocked in CAS-Block o/n at 4°C with gentle rocking. Next day, tissues were incubated in primary antibody in CAS-Block + 0.5%TritonX o/n at 4°C with gentle rocking. Tissues were then washed in PBS+0.1%Tween20 5 times for 1 hour each at RT, incubated in secondary antibody in CAS-Block + 0.5%TritonX o/n at 4°C and next day washed again in PBS+0.1%Tween20 5 times for 1 hour each at RT. Next, tissues were dehydrated to 100 % Methanol through a series of 10 min washes in 25%, 50% and 75% Methanol. Following washes in 100% Methanol three times at RT, tissues were mounted in BABB (1/3 Benzyl alcohol, 2/3 Benzyl Benzoate) on concave well slides (Electron Microscopy Sciences).
Antibodies
The following primary antibodies were used on sections at 1:100 dilution unless otherwise indicated: Claudin5 (Santa Cruz, sc28670), Connexin40 (Santa Cruz, sc20466), E-cadherin (BD Transduction, 610182), Endomucin (Santa Cruz, sc65495), GFP (Aves Labs, GFP-1020, 1:500), Nrp1 (R&D Sytems, AF566), Nrp2 (Cell Signaling, 3366s), PECAM-1 (BD Transduction, 553370), Podocalyxin (R&D Systems, AF1556), smooth muscle alpha (Abcam, ab14106), VE-Cadherin (Santa Cruz, sc6458), VEGF (Abcam, ab14708), ZO1 (Invitrogen, 339100).
The following primary antibodies were used in whole mount staining at indicated dilutions: Connexin40 (Santa Cruz, sc20466, 1:100), E-cadherin (BD Transduction, 610182, 1:100), Endomucin (Santa Cruz, sc65495, 1:400), GFP (Aves Labs, GFP-1020, 1:500), PECAM-1 (BD Transduction, 553370, 1:400).
Secondary antibodies were used at 1:500 dilution: Alexa goat α mouse-488, Alexa goat α mouse-555, Alexa donkey α mouse-555, Alexa donkey α rabbit-555, Alexa chicken α rat-488, Alexa goat α rat-555, Alexa donkey α goat-555, Alexa donkey α goat-488, Alexa donkey α chicken-488.
Eosin staining on sections
Following paraffin sectioning, slides were de-paraffinized and dehydrated by washing twice in xylene for 6 min each, twice in 100 % Ethanol for 2 min each, twice in 95 % Ethanol for 2 min each. Then, slides were rinsed in water for 5 min and incubated in Eosin solution for 3 min. Slides were then washed in 95 % Ethanol twice for 2 min each, in 100 % Ethanol twice for 2 min each, in Xylene twice for 2 min each and mounted with Permount.
Digoxigenin-labeled RNA probes
cDNA containing plasmid was linearized using a one-cutter restriction enzyme and an antisense Digoxigenin(Dig)UTP-labeled RNA probe was synthesized. Probe synthesis was performed at 37°C for 2 hours: 1μg linearized plasmid, 2.0μl 10X transcription buffer (Roche), 2.0μl DIG-RNA labeling mix (Roche), 1.5μl Placental ribonuclease inhibitor (Promega), 1.0μl T3, T7 or SP6 RNA polymerase (Roche) depending on plasmid, RNase-free water to a final volume of 20μl. DNA was eliminated by adding 2μl RQ1 DNase I (Promega), incubated at 37°C for 15 min. Micro Bio-spin columns (Bio-RAD) were used to purify the RNA probe. 10X hybridization stock was prepared at 10μg/ml by adding the appropriate volume of pre-hybridization solution onto the probe. Pre-hybridization solution recipe is as follows: 50% Formamide (Fisher), 5XSSC (pH 4.5), 50μg/ml Ribonucleic acid from Torula yeast, Type VI (Sigma), 1% SDS, 50μg/ml Heparin (Sigma).
Dig-RNA probes were generated using clones from Open Biosystems, unless indicated otherwise: APJ (BC039224), CoupTFII (BC094360), Connexin40 (BC053054), Dll4 (BC042497), Endoglin (BC029080), Endomucin from Invitrogen (BC003706), ICAM2 (BC039128), Nrp1 (BC060129), Nrp2 (BC098200), Pecam-1 (BC008519), Rasip1 as described before (Xie et al., 2009), Sox18 (BC006612).
Whole mount in situ hybridization
Following fixation, whole mount in situ hybridization was carried out using a protocol adapted from D. Wilkinson’s Method (Wilkinson, 1999). Briefly, tissues stored in 70% ethanol at −20°C were rehydrated through a series of Ethanol washes to PBST. Next, the embryos were treated with 10 μg/ml proteinase K at RT (15-30min, depending on the embryonic stage), fixed in 0.2% gluteraldehyde/4% paraformaldehyde for 20 min at RT, and pre-hybridized at 65°C for 1 hour. The samples were transferred into hybridization mix, containing 1μg/ml Dig-labeled probes described above, and incubated o/n at 65°C. Development of color reaction was performed using BM Purple (Roche). Images were taken using a Lumar dissecting microscope (Zeiss) and a DP-70 camera (Olympus).
In situ hybridization on sections
Paraffin sections on slides were de-paraffinized in xylene by washing twice for 10 min each. Slides were hydrated through a series of 100%, 95%, 90%, 80%, 70% and 40% Ethanol washes for 1 min each. Then slides were washed in PBS, followed by a 10min treatment with 15μg/ml proteinase K. Sections were rinsed in PBS and fixed in 4% PFA for 5 min. Pre-hybridization was carried out in pre-hybridization buffer for 1 hour at 65°C. Slides were then transferred to a humidified chamber (with 50% formamide/5X SSC) for probe hybridization. Hybridization was carried out with coverslips on the slides at 65°C o/n. Next day, slides were rinsed in 5X SSC at 65°C to allow coverslips to separate. Then, slides were washed twice in 0.2X SSC for 30 min each at 65°C and once for 5 min at RT. Slides were then transferred to MBST buffer (100mM Maleic acid, 150mM NaCl, pH7.5, 0.1% Tween20). Slides were incubated in blocking solution (2% blocking reagent from Roche in MBST) for 1 hour at RT. Anti-Dig alkaline phosphatase conjugated antibody (Roche) was applied at 1:4000 onto slides in a humidified chamber (with MBST). Slides were covered with parafilm and incubated o/n at 4°C. Next day, slides were washed three times for 30 min each in MBST and three times for 5 min each in NTMT (100mM NaCl, 100mM Tris pH9.5, 50mM MgCl2, 0.1% Tween20). BM purple was used for color reaction as described above. Slides were mounted using Permount and cover-slipped.
β-Galactosidase reaction
For β-Galactosidase reaction on whole mount, tissues were fixed in 5mM EGTA (pH 8.0), 0.2% gluteraldehyde, 2mM MgCl2 in PBS for 15 min on ice. Following fixation, tissues were rinsed in PBS three times for 5 min each. LacZ staining solution was prepared as follows: 20 mM K4Fe(CN)6·3H2O, 20 mM K3Fe(CN)6, 2 mM MgCl2, 0.02% NP40 in PBS. The staining solution was warmed to 37°C before adding X-Gal to avoid precipitation. X-Gal solution (in dimethyl formamide) was then added to the lacZ staining solution at a final concentration of 0.8 mg/ml. Tissues were incubated in the final staining solution at 37°C o/n. When staining was optimal, tissues were rinsed in PBS three times for 5 min each and post-fixed in 4%PFA o/n. Tissues were then placed in 80% glycerol for microscopy.
For β-Galactosidase reaction on sections, the reaction was carried out on whole mounts and then the tissues were paraffin-embedded and sectioned.
RESULTS
Blood vessel integration into the initially avascular pancreatic epithelium
The dorsal pancreatic bud evaginates from the gut tube between E8.75-E9.0 adjacent to the dorsal aorta, and comes to lie immediately anterior to the portal vein at E10.5 (Pan and Wright, 2011). The pancreatic primordium then undergoes extensive morphogenesis to give rise to a highly branched, tubular epithelium. It remains unclear how the pancreas coordinately grows with its associated vasculature as it branches and expands. To understand this process, we analyzed when and where the pancreatic vasculature emerges with respect to the developing epithelium, at different developmental stages. Sections of embryonic pancreas were immunostained for endothelial PECAM-1 and epithelial E-cadherin, starting at E9.5. In parallel, Flk1-lacZ and Flk1-GFP lines were used to identify ECs, as well as angioblasts. These vascular reporters allowed spatio-temporal analysis of the initial specification of ECs, as well as their subsequent morphogenesis into mature vessels.
Small capillaries were observed within the mesenchyme around the periphery of the pancreatic bud epithelium early at E9.5 during bud initiation (Figure 1A). These capillaries often appeared in direct contact with the epithelium (Figure 1A). No arteries were distinguishable at this stage, as the arterial marker Connexin40 did not label vessels in the plexus surrounding the pancreatic bud (data not shown). At E10.5-E11.0, blood vessels continued to be located around (but not in) the stratified pancreatic bud epithelium, which remained avascular, as previously described (Pierreux et al., 2010). This is shown using anti-PECAM-1 staining (Figure 1B) and the Flk1-lacZ reporter line (Figure S1A). At E11.5-E12.0, the pancreatic epithelium begins to remodel, forming bulging ‘tips’ along its entire periphery (Pan and Wright, 2011). As these epithelial branches emerged, blood vessels became intercalated between them, as shown by E-cadherin along with PECAM-1 staining or β-galactosidase activity on Flk1-lacZ embryos (Figure 1C and S1B). Three-dimensional (3D) reconstructions of whole mount PECAM-1 (or PECAM-1/Endomucin costaining, termed ‘PE’) staining along with E-cadherin revealed the morphogenetic events at E10.5-E11.5 underlying epithelial-vascular integration (Figure S1C–D, Supplementary Movie 1–2). Interestingly, blood vessel sprouts were almost never observed, and the initial intercalation seemed to result from epithelial growth into the mesh-like network of surrounding capillaries (Supplementary Movie 1). We also noted that capillaries surrounding the E10.5 bud possessed blood flow, as suggested by presence of auto-fluorescent blood cells within lumens (Figure S1C, arrows). At later stages, pancreatic epithelial ‘tips’ branched and extended further, coordinately growing along with their vasculature, resulting in a highly vascularized branched organ (Figure 1D).
Figure 1. Vascularization of the developing pancreas through intercalation with epithelial branches.
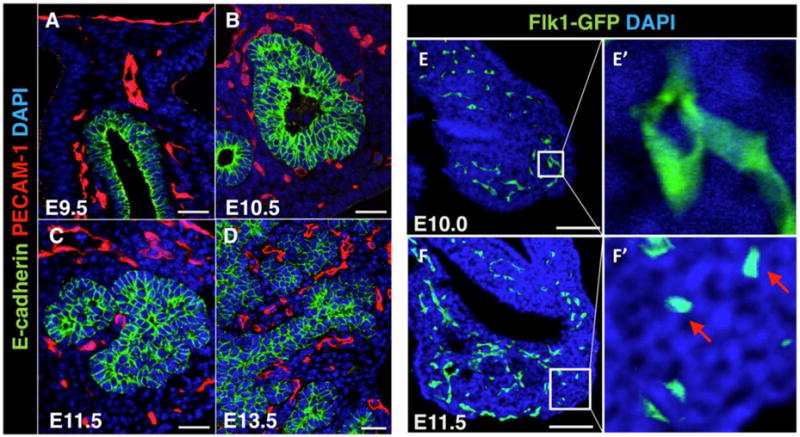
(A–D) Paraffin sections of E9.5-13.5 pancreata immunostained for epithelial membrane marker E-cadherin (green) and endothelial marker PECAM-1 (red). DAPI (blue) marks nuclei. Scale bars 20 μm. (E–F′) Cryosections of E10.0 and E11.5 Flk1-GFP pancreata immunostained with GFP antibody. Squares in (E, F) indicate regions shown at high magnification in (E′, F′). Arrows in (F′) indicate isolated angioblasts. DAPI (blue) marks nuclei. Scale bars 100 μm.
We utilized the Flk1-GFP line to analyze the vascular network in the early pancreatic mesenchyme at a higher resolution. Presence of small capillaries around the stratified bud was validated using this line (Figure 1E–E′). Additionally, putative isolated and rounded angioblasts were identified at E11.5 in the distal pancreatic mesenchyme (Figure 1F–F′). Whole mount staining of Flk1-GFP embryos labeled isolated EC precursors at this stage, as well, suggesting that vessels in the distal pancreas form, at least partially, via vasculogenesis, as previously suggested (Figure S2A–F) (Drake, 2003). These findings indicate that the early pancreas gets vascularized through a combination of epithelial growth into a pre-existing mesenchymal vascular plexus of patent capillaries and peripheral vasculogenesis.
Pancreatic epithelium expresses VEGF
Vascularization in the embryo is known to be directed by numerous vasculogenic factors. VEGF is a very potent such factor expressed by most epithelia during organogenesis. To further evaluate vascularization of the developing pancreas, we examined VEGF expression during pancreas development using the transgenic reporter VEGF-lacZ. β-galactosidase expression was observed at E11.5 throughout the pancreatic epithelium by whole mount staining and on sections (Figure S3A–B). Thus, peripheral vasculogenesis in the developing pancreas may be directed by early epithelial expression of VEGF. At later embryonic stages, the pancreatic epithelium heterogeneously expressed VEGF (Figure S3C–D). Developing endocrine islets exhibited high VEGF expression (Figure S3E–E′, dashed lines). By contrast, acini at the epithelial tips were devoid of reporter expression (Figure S3E–E′, arrows). This lack of VEGF expression at epithelial tips was previously reported with in situ hybridization for VEGF mRNA (Pierreux et al., 2010). The ductal trunk epithelium exhibited high VEGF expression, similar to islets (Figure S3F–F′, arrowheads). VEGF and VEGFR2 antibody staining on adult pancreata also revealed high VEGF expression and advanced vascularization in islets, respectively (Figure S3G–H) (Lammert et al., 2003). This expression pattern of VEGF is in line with the reported heterogeneous vascularization of the pancreas at the corresponding stages, where islets and ducts are closely associated with ECs, whereas acini are devoid of vasculature (Pierreux et al., 2010).
Pancreatic vasculature remodels from plexus to hierarchical vessels
At E9.5, scattered β-galactosidase expression was observed in the pancreatic mesenchyme of Flk1-lacZ embryos, indicating presence of isolated angioblasts (Figure 2A). Slightly later, a primitive primary plexus developed in the mesenchyme surrounding the pancreatic epithelium at E10.5 (Figure 2B, C). The vessels in this plexus were of uniform size and formed a honeycomb-like network that surrounded the gut tube like a net, including the pancreatic bud (dashed line in Figure 2C). The existence of this plexus at E10.5 preceded integration of epithelial branches with blood vessels at E11.5, consistent with the idea that epithelial branches grow into a pre-existing mesenchymal mesh-like vasculature. As development proceeded, we observed vessels aligning and coalescing along the pancreatic bud. A larger central vessel formed along the proximodistal axis of the pancreas, between E11.0 and E11.5 (Figure 2D–E, yellow arrows). This centrally-located pancreatic vessel was consistently present by E12.5 in all embryos examined (Figure 2F, yellow arrow). Therefore, the pancreatic plexus remodeled as its first main vessel formed.
Figure 2. Vascular remodeling in the pancreas: from plexus to hierarchical vessels.
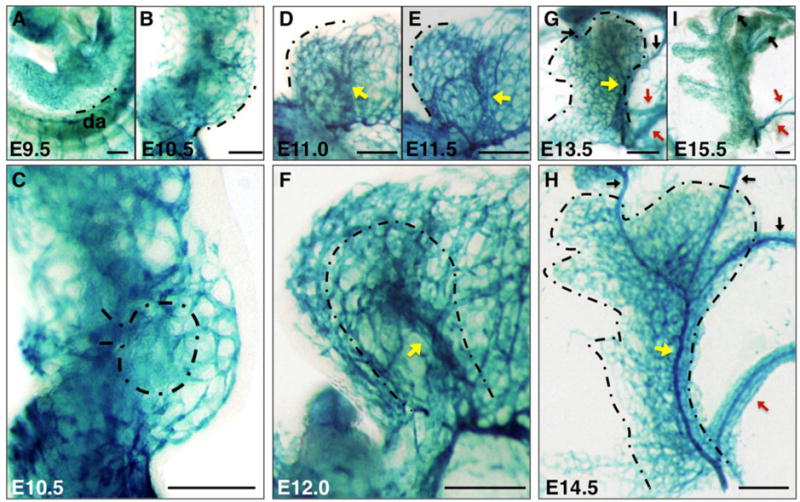
Flk1-lacZ pancreata at indicated stages stained for β-Galactosidase activity in whole mount to label endothelial cells and angioblasts. (A, B, D, E) Dashed line marks the same region along the pancreatic boundary throughout development for convenience. (C, F, G, H) Dashed line represents the boundary of pancreatic bud. (D–H) Yellow arrows indicate the developing large central vessel along the proximodistal axis of the pancreas at E11.0-14.5. Red arrows designate large vessels connecting the pancreas to the spleen along the distal pancreas at E13.5-15.5, and black arrows indicate vessels extending from proximal pancreas to the stomach at E13.5-15.5. Scale bars 200 μm. da, dorsal aorta.
By E13.5, the central vessel became progressively integrated into the expanding pancreas, changing its relative position from the periphery to more internal regions within the pancreas, likely as a consequence of relative epithelial branch outgrowth (Figure 2G, yellow arrow). This remodeling vessel narrowed by E14.5, yet became a prominent structure contiguous with the rest of the pancreatic capillary bed (Figure 2H, yellow arrow). Additionally, as the spleen took shape within the stomach mesenchyme, a few larger vessels appeared along the distal pancreas connecting the pancreas to the spleen, likely as a result of plexus remodeling in this region (Figure 2G–I, red arrows). Similarly, other vessels extended from the proximal pancreas to connect directly to the stomach (Figure 2G–I, black arrows). These observations were corroborated using an alternative vascular maker, PECAM-1, at E9.5–E12.0 (Figure S4).
Early specification of pancreatic arteries and veins
The two major vessel types that carry out blood circulation, arteries and veins, are known to be established early during development in the mouse embryo (Chong et al., 2011). To determine where and when arteries and veins of the pancreas arise, we utilized EphrinB2-lacZ and EphB4-lacZ mouse lines that delineate arteries and veins, respectively. Surprisingly, arteriovenous domains could be distinguished in the mesenchyme surrounding the pancreas as early as E10.5. At this stage, the pancreatic bud extends dorsal to the gut tube at the embryonic midline. Vascular plexuses expressing EphrinB2-lacZ could be observed in the anterior mesenchyme, towards the stomach (Figure 3A). By contrast, vascular plexuses expressing EphB4-lacZ were restricted to the posterior mesoderm, extending from the portal vein region (Figure 3B). No large vessels were identified expressing either marker at this early stage. Therefore, although arteriovenous markers are expressed in angioblasts of the early pancreatic mesenchyme, arteriovenous differentiation does not occur until after E10.5.
Figure 3. Artery and vein specification in the developing pancreas.
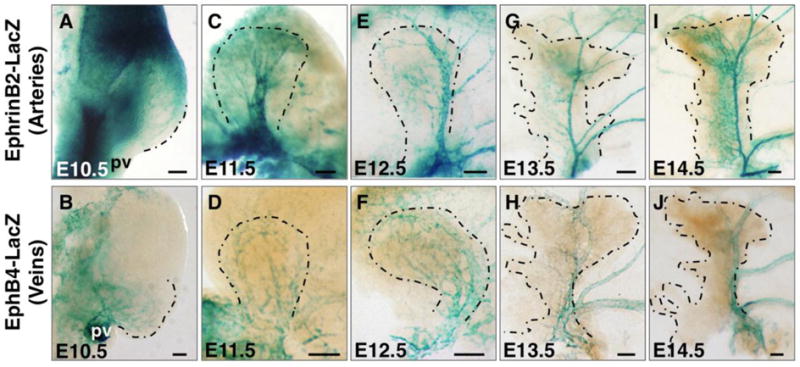
(A, C, E, G, I) EphrinB2-lacZ pancreata at indicated stages stained for β-Galactosidase activity in whole mount to label arteries. (B, D, F, H, J) EphB4-lacZ pancreata at indicated stages stained for β-Galactosidase activity in whole mount to mark veins. Dashed line represents the boundary of pancreatic bud. Scale bars 100 μm. pv, portal vein.
Arteries and veins emerge at distinct locations
By E11.5, the larger central vessel described in Figure 2 could be distinguished as an artery, as it expressed EphrinB2-lacZ (Figure 3C). Veins expressing EphB4-lacZ formed a primitive plexus along the bud periphery at this stage (Figure 3D). By E12.5, the central artery had already established a ramifying hierarchy of large and small vessels (Figure 3E), whereas the venous vessels appeared to be enlarged, a hallmark of vessel remodeling (Figure 3F). A thin central artery and a thicker large vein could be distinguished at E13.5 (Figure 3G, H). By E14.5, most vessels appeared resolved and comprised a network of large and small arteries and veins (Figure 3I, J). These data suggest that in the developing pancreas, similar to the early embryo, arteries are established before veins (Chong et al., 2011). Moreover, arteries and veins arise early and become established at predictable and apposed locations within the pancreas; with veins localizing at the periphery, and arteries more internally.
Dynamic arteriovenous gene expression in pancreatic vessels
To further characterize the main vessels within the developing pancreas at the molecular level, we performed in situ hybridization using established arteriovenous markers. No expression of these genes was found in main vessels of the pancreatic bud at E10.5, in line with our observations using EphrinB2-lacZ and EphB4-lacZ mouse lines (Figure 4A–F). The artery marker Connexin40 initiated in the differentiating central artery at E12.5, in a pattern similar to EphrinB2 (Figure 4A–A″). By contrast, the arterial markers Neuropilin1 (Nrp1) and Dll4 were expressed more diffusely in smaller peripheral vessels, and only transiently marked the central artery (Figure 4B–B″, and C–C″).
Figure 4. Dynamic arteriovenous gene expression in the developing pancreas.
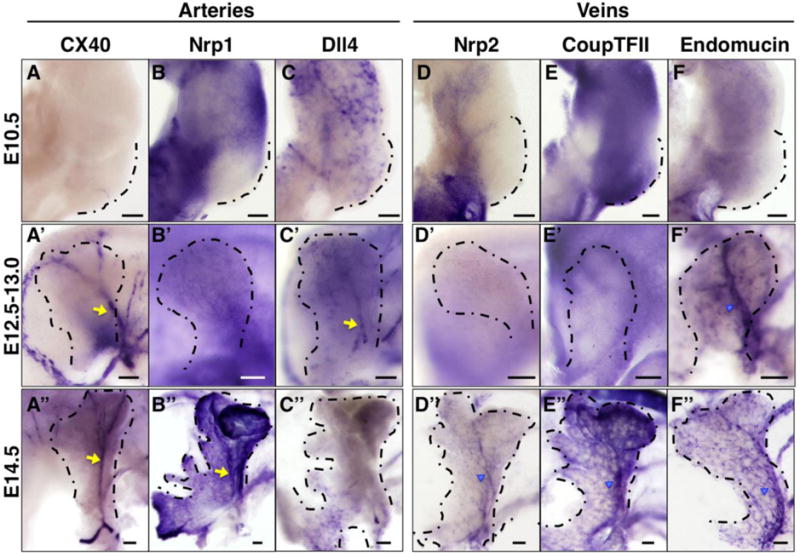
Whole mount in situ hybridization on embryonic pancreata for artery markers (A–C″, yellow arrows) and vein markers (D–F″, blue arrowheads) at indicated stages. Dashed line represents the boundary of pancreatic bud. Scale bars 100 μm.
Expression of venous markers exhibited a generally later onset. Endomucin showed expression in the central vein, while Neuropilin2 (Nrp2) and CoupTFII were absent from this vessel initially, at E12.5 (Figure 4D–F″). Later, all three became expressed as the vein differentiated (Figure 4D″, E″ and F″). Of note, unlike EphB4, none of these markers were restricted to the main vein. Thus, EphrinB2 and Connexin40 represent the only markers restricted to the main central artery, while EphB4 is the only one restricted to the main central vein, among the gene expression profiles we tested. Overall, both main vessels in the developing pancreas exhibit temporally dynamic molecular signatures.
Central pancreatic artery ECs become cuboidal following capillary coalescence
To determine how the main artery in the pancreas forms following vascular remodeling, we analyzed this process by whole-mount stains and high-resolution immunofluorescence imaging. Connexin40 whole mount immunostaining revealed multiple parallel, and likely coalescing, vessels at E11.5, in the region where the prospective central artery develops (Figure 5A, arrows). This pattern was similar to that of EphrinB2-lacZ (Figure 3C). These data suggest that arterial fate precedes vessel coalescence. Sections of Flk1-GFP pancreata stained for Connexin40 also marked multiple capillaries in contact with each other, likely in the process of coalescing (Figure 5A′). These capillaries had lumens, as suggested by the presence of blood in identifiable lumen spaces (Figure 5A′, lumens indicated by dashed lines, Figure S5A).
Figure 5. Development of the central artery in the pancreas via vessel coalescence.
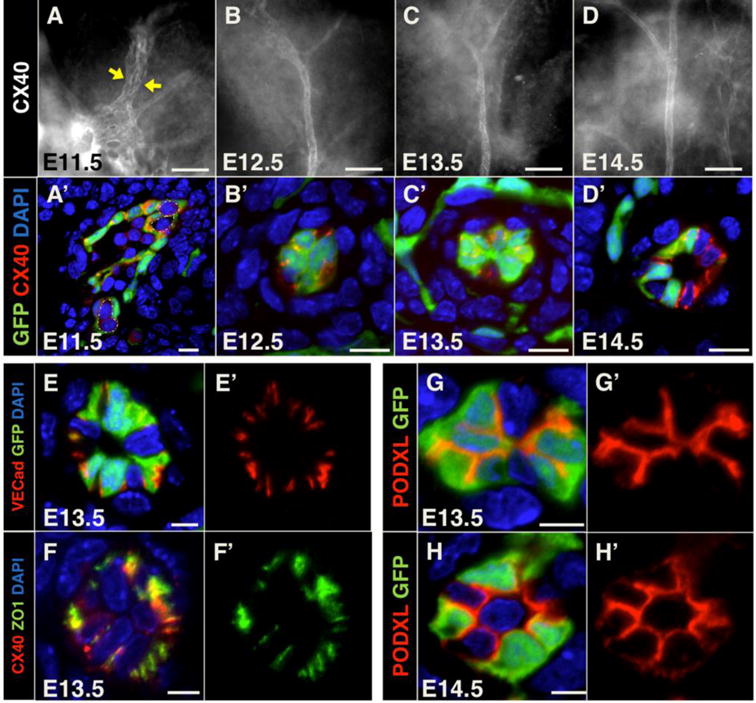
(A, B, C, D) Whole mount pancreata at indicated stages immunostained for the artery marker Connexin40 (CX40). Yellow arrows indicate coalescing vessels marked by Connexin40. Scale bars 50 μm. (A′, B′, C′, D′) Cryosections of Flk1-GFP pancreata at indicated stages immunostained for GFP (green) and Connexin40 (red) to mark endothelial cells and arteries, respectively. Dashed lines in A′ represent lumen boundaries. Scale bars 10 μm. (E–E′) Cryosections of E13.5 Flk1-GFP pancreata immunostained for GFP (green) and the endothelial junctional marker VE-cadherin (red). Scale bars 5 μm. (F–F′) Cryosections of E13.5 pancreata immunostained for the tight junction molecule Zona Occludens 1 (ZO1, green) and Connexin40 (red). Scale bars 5 μm. (G–H′) Cryosections of E13.5 and E14.5 Flk1-GFP pancreata immunostained for GFP (green) and the apical glycoprotein PODXL (red). Scale bars 5 μm. DAPI (blue) marks nuclei when applicable.
Interestingly, as the nascent artery formed, ECs became cuboidal and the vessel became constricted (Figure 5B–C′). In fact, the vessel appeared nearly closed along most of its length, throughout the longitudinal axis of the pancreas (Figure 5B, C). At E14.5, the lumen of the central artery opened progressively from proximal to distal pancreas, as its ECs reacquired a squamous morphology (Figure 5D–D′). Of note, the cells of the nascent artery heterogeneously expressed Flk1-GFP (Figure 5B′, C′, D′). Staining with VE-Cadherin or ZO1 antibodies further revealed that arterial ECs did not seal any luminal cavity at E13.5, as these junctional markers localized peripherally (Figure 5E–F′). Assessment of apical Podoclayxin (PODXL) in ECs of the artery indicated that EC polarity is maintained during lumen constriction (Figure 5G–H′). Interestingly, smooth muscle 22 alpha (SM22α) positive cells were observed to be recruited to coalescing arterial vessels and to wrap around the central artery starting at E13.5 (Figure S5B–D′). This timing indicates that mural cells are recruited, not as the lumen constricts and ECs become cuboidal, rather from the onset of vessel formation and as the lumen begins to open. Thus, the main central artery in the pancreas arises following coalescence or fusion of vessels, and its ECs undergo significant cellular shape changes as they develop a lumen.
Heterogeneity in the developing pancreatic vascular bed
Given that established artery and vein markers display heterogeneous expression patterns during embryonic development (Chong et al., 2011), as well as pancreas development, we further assessed EC heterogeneity in the capillary beds of the growing bud. We performed in situ hybridization for known vascular genes at three different stages: E10.5, E12.5 and E14.5. Candidates were chosen following a www.genepaint.org screen for genes enriched in pancreatic blood vessels (using advanced search function). We found that vessels of the developing pancreas are highly heterogeneous. At E10.5, gene expression appeared either punctate (Sox18, ICAM2 and PECAM-1; Figure S6A–C) or in net-like patterns (APJ, Endoglin and Rasip1, Figure S6D–F). Furthermore, some genes showed expression in the periphery (Sox18, APJ, Endoglin, Rasip1; Figure S6A, D, E and F), while others were absent in this region (ICAM2 and PECAM-1; Figure S6B and C). At later stages, a subset of markers were found to be expressed in the central artery (ICAM2 and PECAM-1; arrows, Figure S6B′, B″, C′ and C″), in the main vein (Endoglin; arrows, Figure S6E′ and E″), or in both (Rasip1; arrows, Figure S6F′ and F″). Moreover, several markers were enriched in the tissue interior (Sox18, PECAM-1; Figure S6A″ and C″), whereas others appeared uniformly expressed throughout the pancreas (APJ and Endoglin; Figure S6D″ and E″). Heterogeneity of endothelial marker distribution is illustrated with whole mount tissues and sections, showing Flk1-LacZ, APJ and Rasip1 expression (Figure S7A, B, D, E, G, H). Schematics further compare their expression and show the differences in expression patterns between vascular markers (Figure S7C, F, I). Thus, the pancreas develops coordinately with highly heterogeneous and evolving vascular beds.
Over-expression of pancreatic VEGF disrupts pancreatic vascular patterning
We next asked how pancreatic blood vessels respond to classical vascular disruptions, by examining alterations in vessel architecture, gene expression and arteriovenous fate. Pdx1-driven VEGF over-expression (Pdx1-tet-VEGF) has been reported to cause hypervascularization and thereby result in decreased epithelial differentiation, branching and growth (Magenheim et al., 2011a). However, beyond overgrowth of vessels, effects of excess VEGF on the pancreatic vascular architecture have not been explored. Here, we examined vascular development in the pancreas in the presence of excess VEGF.
We found that indeed the vascular architecture was significantly altered in double transgenic embryos at E12.5, compared to controls. Single transgenic Pdx1-tTA or TET-VEGF embryos exhibited no phenotype and were used as controls. The pancreatic central artery was marked by Connexin40 at E12.5 and could easily be distinguished from other vessels in controls (Figure 6A, red stippled lines). By contrast, multiple smaller vessels were marked by Connexin40 in this region in Pdx1-tet-VEGF pancreata (Figure 6A′, red stippled lines). In addition, irregular branching replaced the predictable arrangement of a single central artery and smaller, branched vessels (Figure 6A′). In order to identify any effect on vascular heterogeneity, we utilized the endothelial markers APJ, Rasip1 and Endoglin, which exhibited heterogeneous expression in pancreatic vascular beds as demonstrated in Figure S6. APJ in situ hybridization revealed abnormal venous network in the region of the main vein in Pdx1-tet-VEGF pancreata, while the peripheral honeycomb-like organization of APJ expressing vessels was lost (Figure 6B–B′). Similarly, expression of Rasip1 and Endoglin were expanded into a disordered vascular network in the pancreatic mesenchyme upon VEGF over-expression (Figure S8AB′). Furthermore, ectopic branches extended from the artery that connects pancreatic mesenchyme to the spleen as marked by Connexin40, APJ and Rasip1 (Figure 6A′, B′, Figure S8A′).
Figure 6. Excessive pancreatic VEGF leads to distinct patterning defects in different vascular beds.
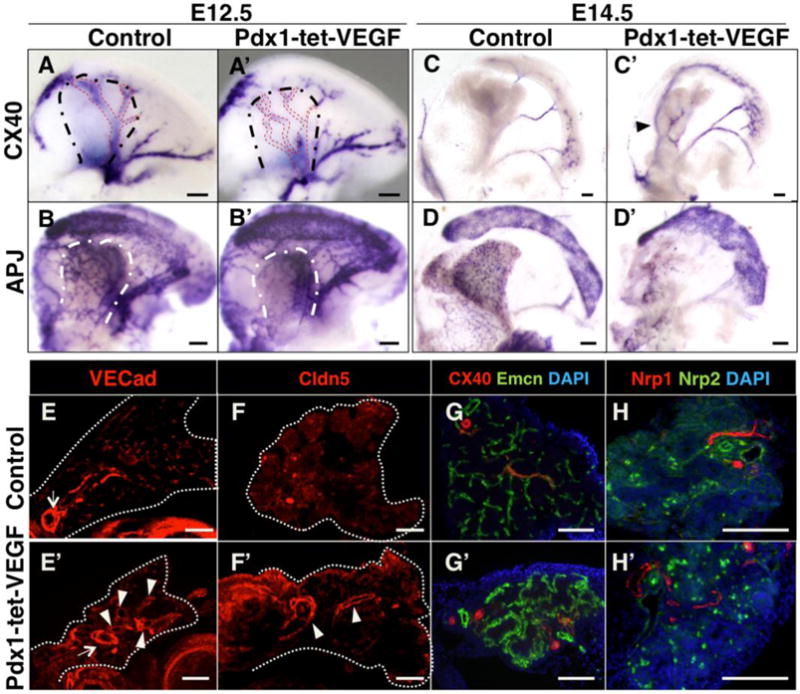
(A–D′) Whole mount in situ hybridization of E12.5 and E14.5 control or Pdx1-tet-VEGF pancreata for Connexin40 and APJ as indicated. (E–H′) Immnunostaining on paraffin sections of E14.5 control or Pdx1-tet-VEGF pancreata with indicated antibodies. (A–A′) Black dashed lines mark the boundaries of pancreatic bud. Red dashed lines represent vessels marked by Connexin40 expression. Note ectopic vessels with abnormal architecture in E12.5 Pdx1-tet-VEGF pancreas. (B–B′) White dashed lines represent boundaries of pancreatic bud. Abnormally dense and narrow APJ expression domain can be identified in E12.5 Pdx1-tet-VEGF pancreata. (C–C′) Arrowhead indicates an ectopic large vessel with ectopic branches in E14.5 Pdx1-tet-VEGF pancreas not present in control. (D–D′) Reduced number of vessels with abnormal architecture is marked by APJ in E14.5 Pdx1-tet-VEGF pancreas, as opposed to peripheral mesh-like expression in controls. (E–F′) Arrows indicate a main vessel present in both controls and Pdx1-tet-VEGF pancreata. Arrowheads designate ectopic vessels in Pdx1-tet-VEGF pancreas. Both endothelial junctional markers, VE-cadherin (VECad) and Claudin5 (Cldn5), mark ectopic vessels. (G–G′) Ectopic vessels in the Pdx1-tet-VEGF pancreas express the arterial marker Connexin40 (red). In Pdx1-tet-VEGF buds, the vascular bed that expresses the venous marker Endomucin (Emcn, green) contains vessels of uniform size, in contrast to controls, which consist of ramifying vessels, with centrally located large vessels and peripheral capillaries. (H–H′) Immunostaining with the artery marker Nrp1 (red) identifies ectopic vessels, while the vein marker Nrp2 (green) labels a reduced number of ECs in the Pdx1-tet-VEGF pancreata (round cells are autofluorescent blood). All scale bars 100 μm.
By E14.5, the pancreata of Pdx1-tet-VEGF transgenics appeared smaller and irregular, while the architectural abnormalities of the pancreatic vasculature became more pronounced. Connexin40 marked ectopic large vessels with branches in Pdx1-tet-VEGF pancreata, while these vessels were not marked in controls (Figure 6C–C′, arrowhead). Additionally, the central artery lacked several branches (Figure 6C–C′, and Figure S8C–C′), and its bifurcation point was more proximal than in controls (Figure S8C–C′, arrows). Ectopic Connexin40 expressing vessels in Pdx1-tet-VEGF pancreata were also observed on sections (Figure S8D–D′, arrowheads). Similar to E12.5, examination of APJ expression identified a severely disordered vascular bed in the pancreatic mesenchyme (Figure 6D–D′). The number of cells expressing APJ was reduced along the bud periphery in VEGF over-expressing embryos, but was abnormally concentrated along the pancreatic ridge (raised structure along the dorsal pancreas) (Figure S8E–E′, arrowhead), in line with its aberrantly concentrated mRNA expression at E12.5.
Arteriovenous architectural abnormalities in Pdx1-tet-VEGF pancreata could also be identified by immunostaining with specific markers. Ectopic large vessels were observed using the endothelial markers VE-Cadherin and Claudin5 (Figure 6E–F′, arrowheads). Connexin40 and Nrp1 immunostaining also labeled ectopic large vessels, confirming the in situ hybridization data and indicating formation of ectopic arteries (Figure 6G–H′). The vein marker Endomucin revealed an abnormal coalescence of capillaries with uniformly sized vessels in double transgenics, as opposed to the more centrally-located, fewer and larger vessels in controls (Figure 6G–G′). This suggests that the venous bed lost its hierarchical organization following VEGF over-expression. Additionally, a decreased number of ECs expressed the venous marker Nrp2 in the Pdx1-tet-VEGF pancreas, relative to controls (Figure 6H–H′). These data indicate that Pdx1-tet-VEGF pancreata develop ectopic arteries and non-hierarchical, closed venous networks in addition to abnormal peripheral and interior capillary beds, as marked by APJ and Rasip1, respectively. Thus, the vascular architecture of the developing pancreas is exquisitely sensitive to proper levels of VEGF over-expression.
DISCUSSION
Pancreas development has been extensively studied over the years, particularly in the context of beta cell differentiation, driven by the need to develop better diabetes therapies. More recent studies showed that blood vessels regulate beta cell biology in various ways, both during early differentiation as well as later beta cell maturation and function (Brissova et al., 2006; Cai et al., 2012; Katsumoto and Kume, 2011; Lammert et al., 2001; Magenheim et al., 2011b; Pierreux et al., 2010; Yoshitomi and Zaret, 2004). However, how and when vascularization of the developing pancreas is initiated, and how the pancreatic vasculature takes shape, have remained open, yet basic, questions. In this study, we provide an in-depth characterization of early pancreatic vascular development. Specifically, we demonstrate early blood vessel ingression as a mechanism for vascularization of the embryonic pancreas, followed by a step-wise progression of vascular remodeling and arteriovenous differentiation. We also examine the abnormalities in vascular architecture caused by VEGF over-expression in the pancreas, and demonstrate that the effects are distinct depending on the vascular bed. Our findings will forward our understanding of pancreas development, as well as how the embryo coordinates blood vessel and organ formation.
Pancreas vascularization by vessel intercalation
Our examination of early pancreatic vasculature yielded unexpected findings. We found no evidence of angiogenic sprouting into the pancreatic epithelium, as might have been expected given observations in other developing tissues where angiogenic sprouting drives vascularization, such as in the neural tube, retina, heart and CNS (Acker et al., 2001; Gerhardt et al., 2003; Hogan et al., 2004; Kurz et al., 1996; Red-Horse et al., 2010). Rather, our data supports the idea that epithelial growth passively drives vascular integration and intercalation into the pancreas, as the epithelium branches into and beyond the surrounding capillary bed. We show that this integration initiates around E11.5-12.5 when branching starts. Others have reported this process during later pancreatic outgrowth from E13.5 (Pierreux et al., 2010). On the other hand, we found widespread peripheral angioblasts in the early pancreatic bud mesenchyme, suggesting that the pancreatic vasculature partially arises from de novo vasculogenesis. Peripheral vasculogenesis, combined with angiogenesis in the tissue interior, has been proposed to form the vascular beds in many organ primordia, including lung and kidney (Drake, 2003). Therefore, it is likely that peripheral vasculogenesis contributes to pancreas-specific vascular beds as the organ expands. Furthermore, our results indicate that capillaries surrounding the E10.5 pancreatic epithelium are perfused with blood, in line with previous findings (Shah et al., 2011). The study by Shah et al. showed that only a subset of capillaries are perfused, and that perfusion correlates with the differentiation state of the epithelium in the vicinity. Thus, we propose that the embryonic pancreas becomes vascularized through integration with partially perfused capillaries and peripheral vasculogenesis.
Pancreas arteriovenous differentiation occurs upon vascular remodeling
Subsequent to these events, we show that the pancreatic vasculature undergoes remodeling to yield arteries, and later veins. What drives this remodeling remains unknown. Interestingly, we note that remodeling of the pancreatic vasculature coincides with gut turning (E11.0) and breaking of gut organ symmetry, suggesting that mechanical forces during this process may be important for pancreatic vascular remodeling. Mechanical force has been shown to govern tissue morphogenesis in many instances, such as in the intestine (Shyer et al., 2015). Further studies of the remodeling process are required to test this idea, or to reveal whether other factors, potentially epithelium-derived signaling mechanisms, are involved.
Arteriovenous differentiation is a major step necessary to form functional vasculature. Here, we demonstrate that, similar to vessels in the early embryo, this differentiation occurs in a step-wise manner in the developing pancreatic vasculature. Our analysis also uncovers several important phenomena: First, arteriovenous specification does not occur simultaneously throughout embryonic tissues. While arteriovenous markers delineate arteries and veins in the E9.5 embryonic trunk, these are expressed in a scattered manner in the pancreatic mesenchyme. Second, similar to vessels in the early embryo, arteries are established prior to veins in the pancreas. It remains to be determined whether this common theme is a pure consequence of the artery/vein differentiation program, or of blood flow, or other regulatory mechanisms are at work. Third, the pancreatic arteries and veins form at predictable, but non-stereotypic, locations. In the developing pancreatic bud, veins appear more physically distant from the epithelium than arteries. The consequences of this differential arrangement require further study from the perspective of epithelial development.
The observation that vessels only express subsets of arteriovenous markers seems to contradict the idea that these genes represent definitive “markers” of arteries and veins. It also argues that arteriovenous fate may not be a simply binary switch. We propose instead that blood vessels in different embryonic organs remodel at distinct developmental timepoints, and express different subsets of markers at different stages of arteriovenous differentiation. These changes in cell fate likely represent local hemodynamic conditions, as blood flow drives both anatomical and transcriptional changes within vascular beds. In addition, we also note that the endothelial reporter Flk1-GFP marked only some cells within the central artery. Although this may result from incomplete penetrance of the transgene, our findings are in line with reported heterogeneity in vasculature, across arteries, veins, or within a single vessel (Aird, 2003).
Central pancreatic artery forms via vascular coalescence
Our study identified the central pancreatic artery as the first organ-specific large vessel arising in this organ. We further demonstrated that this vessel forms via coalescence of capillaries. The observed coalescence process is reminiscent of ramifying large vessel formation in the yolk sac (Lucitti et al., 2007; Udan et al., 2013). How this coalescence is initiated or regulated remain as open questions. One potential factor is blood flow, as hemodynamic forces are known to induce similar processes in vascular remodeling (Hahn and Schwartz, 2009; Lucitti et al., 2007). Unexpectedly, we noticed a drastic cell shape change in ECs of the artery from squamous to cuboidal, following the coalescence process. Cell shape change was coincidental with both mural cell recruitment and lumen closure. Interestingly, ECs of the artery remained polarized normally during all stages of artery differentiation. While we cannot definitively determine whether the artery lumen is functional during this time, we do not observe blood cells within it around E13.0-13.5, when its area is most constricted. Notably, Connexin40 is a blood flow-responsive gene (Vorderwulbecke et al., 2012) and is expressed in the central artery throughout this time. However, this expression is initiated prior to constriction of the vessel (at E11.5) and may continue to be expressed during the transient constriction, even if blood flow is blocked. We speculate that closure of the lumen may re-direct blood flow, away from distal coalescing capillaries, for instance, and that cell shape change is utilized in this process.
Interestingly, we noted that pericytes are in the vicinity of the central artery starting at E11.5, but do not surround the artery until E13.5. It is possible that pericyte-derived signaling regulates the shape of ECs of the pancreatic artery as it forms. Since the pancreatic central artery seems to form at the heart of a remodeling vascular bed, further investigation of this process is likely to provide a better understanding of epithelial-endothelial co-development in the pancreas.
Forming pancreatic vasculature is transcriptionally heterogeneous
Organ-specific capillary beds make intimate contact with cells of tissues and organs, and they are known to serve organ-specific functions (Cleaver and Melton, 2003). Formation of capillary beds in developing organs remains relatively understudied, primarily as a consequence of their size and heterogeneity. Our findings indicate that the developing pancreas has distinct capillary networks that are heterogeneous at the molecular level. Our data suggests that ICAM2 and PECAM-1 mark the relatively more differentiated endothelial beds in the tissue interior, whereas Sox18, APJ, Endoglin and Rasip1 are all expressed in more peripheral, and likely relatively immature, endothelium. These observations suggest that the pancreatic epithelium is nourished by highly heterogeneous capillary beds during its developmental progression.
Potential endothelial influence on pancreatic epithelial development
Endothelium has been shown to regulate epithelial morphogenesis and differentiation in most organs, including hepatocyte polarity in the liver (Sakaguchi et al., 2008). Here, we demonstrate that blood vessel ingression into the epithelium occurs at E11.5, when polarity becomes critical for proper morphogenesis. Whether this temporal correlation translates into a functional relationship remains to be answered. Many studies have also identified a role for blood vessels in regulating pancreatic branching and endocrine differentiation (Magenheim et al., 2011a; Pierreux et al., 2010; Sand et al., 2011a). We investigated the earliest steps in pancreatic vascularization and show that patterning of the vasculature occurs via remodeling of the initial plexus. We also show that the central pancreatic artery forms around E12.5 and we propose that this represents the onset of vascular remodeling in the organ. This time frame coincides with the end of primary transition, when a first wave of primitive endocrine cells delaminates from the pancreatic epithelium. It remains to be determined whether these two events, vascular remodeling and primary transition, are functionally coupled. It is intriguing to hypothesize that the main central artery provides a niche for pancreatic differentiation. Further studies focusing on non-autonomous, circulation-independent functions of the pancreatic endothelium are required to answer these questions.
Excess VEGF disrupts pancreatic vascular patterning through distinct effects on heterogeneous vascular beds
Finally, perturbation of embryonic pancreatic vasculature has been utilized in previous studies to assess indirect effects on epithelial development (Brissova et al., 2006; Cai et al., 2012; Katsumoto and Kume, 2011; Lammert et al., 2001; Magenheim et al., 2011b; Pierreux et al., 2010). However, evaluateon of the findings requires a better understanding of direct effects on pancreatic vasculature.
In this study, we examined vascular development under excessive epithelial VEGF expression in Pdx1-tet-VEGF pancreata. Our data indicate that VEGF over-expressing pancreata have perturbed vascular architecture. Strikingly, not all vessels are affected in the same manner. Ectopic arteries form, while ectopic veins were not observed. This finding is consistent with the role of VEGF in controlling Notch signaling and switching on arterial fate: VEGF over-expression in the zebrafish embryo induces ectopic arterial marker expression, and in the cardiac muscle it results in an increase in arterial, and a decrease in venous, microvessels (Lawson et al., 2002; Visconti et al., 2002). In addition, studies have demonstrated that venous vascular beds depend on BMP signals, rather than VEGF (Wiley et al., 2011). Our observations that pancreatic veins aggregate into irregular networks and lose their hierarchical organization upon VEGF over-expression may result from indirect effects of arterial disruptions, or from dependence of pancreatic venous beds on VEGF. Interestingly, some capillary beds (marked by APJ and Endomucin) coalesce, while others (marked by Rasip1 and Endoglin) expand to an abnormal network in Pdx1-tet-VEGF pancreata. How these differentially perturbed vascular beds contribute to the reported defective epithelial differentiation and morphogenesis phenotypes remains to be explored. Importantly, perturbed epithelial growth and branching in the Pdx1-tet-VEGF pancreata may potentially cause aberrations in vascular development in turn. It remains to be determined whether, or to what extent, pancreatic epithelial abnormalities indirectly cause abnormal endothelial development in these mice, as opposed to direct effects of excessive VEGF expression.
Of note, a recent study identified EGFL7 as a vascular-derived factor that promotes pancreatic progenitor fate (Kao et al., 2015). Given that Pdx1-tet-VEGF pancreata have a block in differentiation, it will be interesting to determine whether EGFL7 is expressed in the ectopically formed arteries or expanded vascular networks of these pancreata. Further studies addressing these questions will expand our understanding of how angiocrine factors affect cell fate decisions and morphogenesis during pancreas development.
In summary, this study provides an in-depth characterization of blood vessel development in the embryonic pancreas, and identifies architectural abnormalities of the vasculature in a classical model of pancreatic vascular perturbation. This work improves the understanding of pancreatic vascular development and thereby provides insight into the factors necessary for proper pancreatic morphogenesis and beta cell differentiation.
Supplementary Material
HIGHLIGHTS.
Initial pancreatic bud epithelium is stratified and avascular.
Branching of the epithelium into surrounding mesenchyme vascularizes the pancreas.
Main pancreatic artery arises as a result of vessel remodeling and coalescence.
Pancreas establishes arteries before veins, as it forms heterogeneous vascular beds.
Arteriovenous patterning depends on regulated VEGF expression.
Acknowledgments
We thank E. Keshet (and J. Rossant) for Flk1(VEGFR2)-lacZ mice; Margaret Baron for Flk1-GFP mice; Andras Nagy for VEGF-lacZ; and Mark Henkemeyer for ephrinB2-lacZ and EphB4-lacZ mice. We are grateful to the MacDonald, Olson, Cleaver and Carroll labs for invaluable discussions and assistance.
FUNDING
This work is funded by NIH Institutional National Research Service Award (T32) 2T32GM008203-26A1 to DBA, and CPRIT RP110405, R01DK079862, R24DK106743 and R01HL113498 to OC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors declare no competing or financial interests.
AUTHOR CONTRIBUTIONS
Experiments were performed by D.B.A, D.C., A.V., D.M.B., L.M.S., S.L., S.F. and J.M. Y.D. and O.C. supervised the project and contributed to the analysis. D.B.A and O.C. wrote the manuscript together, with input from co-authors.
References
- Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev. 2001;108:45–57. doi: 10.1016/s0925-4773(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31:S221–230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A, Poffenberger G, Quaggin SE, Jerome WG, Dumont DJ, Powers AC. Enhanced expression of VEGF-A in beta cells increases endothelial cell number but impairs islet morphogenesis and beta cell proliferation. Dev Biol. 2012;367:40–54. doi: 10.1016/j.ydbio.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DC, Koo Y, Xu K, Fu S, Cleaver O. Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn. 2011;240:2153–2165. doi: 10.1002/dvdy.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- Coveney D, Cool J, Oliver T, Capel B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A. 2008;105:7212–7217. doi: 10.1073/pnas.0707674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69:73–82. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nature reviews. Molecular cell biology. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–1513. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- Kao DI, Lacko LA, Ding BS, Huang C, Phung K, Gu G, Rafii S, Stuhlmann H, Chen S. Endothelial cells control pancreatic cell fate at defined stages through EGFL7 signaling. Stem Cell Reports. 2015;4:181–189. doi: 10.1016/j.stemcr.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto K, Kume S. Endoderm and mesoderm reciprocal signaling mediated by CXCL12 and CXCR4 regulates the migration of angioblasts and establishes the pancreatic fate. Development. 2011;138:1947–1955. doi: 10.1242/dev.058719. [DOI] [PubMed] [Google Scholar]
- Kurz H, Gartner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Lazarus A, Del-Moral PM, Ilovich O, Mishani E, Warburton D, Keshet E. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Werb Z. Patterning mechanisms of branched organs. Science. 2008;322:1506–1509. doi: 10.1126/science.1162783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenheim J, Ilovich O, Lazarus A, Klochendler A, Ziv O, Werman R, Hija A, Cleaver O, Mishani E, Keshet E, Dor Y. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011a doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenheim J, Ilovich O, Lazarus A, Klochendler A, Ziv O, Werman R, Hija A, Cleaver O, Mishani E, Keshet E, Dor Y. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011b;138:4743–4752. doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- Pierreux CE, Cordi S, Hick AC, Achouri Y, Ruiz de Almodovar C, Prevot PP, Courtoy PJ, Carmeliet P, Lemaigre FP. Epithelial: Endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev Biol. 2010;347:216–227. doi: 10.1016/j.ydbio.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B, St John PL, Abrahamson DR. Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am J Physiol. 1998;275:F164–172. doi: 10.1152/ajprenal.1998.275.1.F164. [DOI] [PubMed] [Google Scholar]
- Sakaguchi TF, Sadler KC, Crosnier C, Stainier DY. Endothelial signals modulate hepatocyte apicobasal polarization in zebrafish. Curr Biol. 2008;18:1565–1571. doi: 10.1016/j.cub.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand FW, Hornblad A, Johansson JK, Loren C, Edsbagge J, Stahlberg A, Magenheim J, Ilovich O, Mishani E, Dor Y, Ahlgren U, Semb H. Growth-limiting role of endothelial cells in endoderm development. Developmental biology. 2011a;352:267–277. doi: 10.1016/j.ydbio.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Sand FW, Hornblad A, Johansson JK, Loren C, Edsbagge J, Stahlberg A, Magenheim J, Ilovich O, Mishani E, Dor Y, Ahlgren U, Semb H. Growth-limiting role of endothelial cells in endoderm development. Dev Biol. 2011b;352:267–277. doi: 10.1016/j.ydbio.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SR, Esni F, Jakub A, Paredes J, Lath N, Malek M, Potoka DA, Prasadan K, Mastroberardino PG, Shiota C, Guo P, Miller KA, Hackam DJ, Burns RC, Tulachan SS, Gittes GK. Embryonic mouse blood flow and oxygen correlate with early pancreatic differentiation. Dev Biol. 2011;349:342–349. doi: 10.1016/j.ydbio.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ. Bending gradients: how the intestinal stem cell gets its home. Cell. 2015;161:569–580. doi: 10.1016/j.cell.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan RS, Vadakkan TJ, Dickinson ME. Dynamic responses of endothelial cells to changes in blood flow during vascular remodeling of the mouse yolk sac. Development. 2013;140:4041–4050. doi: 10.1242/dev.096255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasenor A, Cleaver O. Crosstalk between the developing pancreas and its blood vessels: an evolving dialog. Semin Cell Dev Biol. 2012;23:685–692. doi: 10.1016/j.semcdb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci U S A. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderwulbecke BJ, Maroski J, Fiedorowicz K, Da Silva-Azevedo L, Marki A, Pries AR, Zakrzewicz A. Regulation of endothelial connexin40 expression by shear stress. Am J Physiol Heart Circ Physiol. 2012;302:H143–152. doi: 10.1152/ajpheart.00634.2011. [DOI] [PubMed] [Google Scholar]
- Wiley DM, Kim JD, Hao J, Hong CC, Bautch VL, Jin SW. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nature cell biology. 2011;13:686–692. doi: 10.1038/ncb2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. In situ hybidization: A practical approach. 2nd. Oxford University Press; New York: 1999. [Google Scholar]
- Xie J, Wu T, Xu K, Huang IK, Cleaver O, Huang CL. Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice. Am J Pathol. 2009;175:1315–1327. doi: 10.2353/ajpath.2009.090094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


