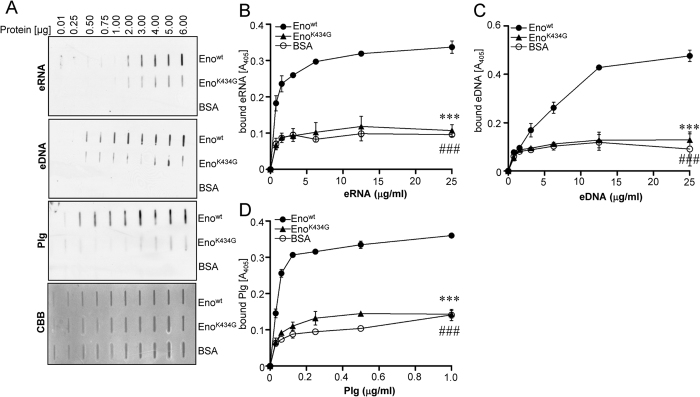
Figure 5. Mutation of Eno C-terminal lysine 434 reduces Eno-eRNA/eDNA interaction.
(A) Bovine serum albumin (BSA), wt pneumococcal Eno (Eno) and Eno mutant (EnoK434G) were spotted on the nitrocellulose membrane. The binding of proteins to eRNA, eDNA and Plg was tested by dot blot. Commassie brilliant blue (CBB) staining was used as a loading control. (B) The binding of pneumococcal Enowt (●), EnoK434G (▲) or BSA (○) to eRNA was determined using the solid-phase binding assay. Immobilized proteins were incubated with different concentration of biotinylated RNA. Eno/eRNA complexes were detected by chemiluminescence. (C) The binding of pneumococcal Enowt (●), EnoK434G (▲) and BSA (○) to eDNA was determined using the solid-phase binding assay. The immobilized proteins were incubated with different concentration of biotinylated eDNA. Eno/eDNA complexes were detected by chemiluminescence. Data represent mean values ± SEM; n = 3; ###p ≤ 0.001; ***p ≤ 0.001 vs wt Eno (●). (D) The binding of Enowt (●), EnoK434G (▲) or BSA (○) to Plg was determined using the solid-phase binding assay. Immobilized proteins were incubated with different concentration of biotinylated Plg. Eno/Plg complexes were detected by chemiluminescence. Data represent mean values ± SEM; n = 3; ###p ≤ 0.001; ***p ≤ 0.001 vs wt Eno (●).