Abstract
Background:
Roughly focused extracorporeal shock waves therapy (ESWT) is characterized by a wide focal area, a large therapy zone, easy positioning, and less pain during treatment. The purpose of this study was to investigate the effects of roughly focused ESWT on the expression of osteoprotegerin (OPG) and bone morphogenetic protein-2 (BMP-2) in osteoporotic fractures in rats.
Methods:
Seventy-two female Sprague-Dawley (SD) rats, 3 months old, were divided into sham-operated group (n = 6) and an ovariectomized (OVX) group (n = 66). Sixty OVX SD rats were used as a model of double proximal tibial osteotomy and inner fixation. The osteotomy site in the left tibia was treated with roughly focused ESWT once at an energy density of 0.26 mJ/mm2, 60 doses/min, and 2000 pact quantities. The contralateral right tibia was left untreated and served as a control. Expression of OPG and BMP-2 in the callus of the osteoporotic fracture area was assessed using immunohistochemistry, real-time polymerase chain reaction (PCR), and Western blotting analysis.
Results:
Bone mineral density (BMD) at the proximal tibia, femur, and L5 spine was significantly reduced after ovariectomy. BMD of proximal tibia was 12.9% less in the OVX group than that in the sham-operated group. Meanwhile, bilateral oophorectomy resulted in a lower trabecular bone volume fraction (BV/TV) in the proximal tibia of the sham-OVX animals. Three months after bilateral oophorectomy, BV/TV was 14.29% of baseline BV/TV in OVX legs versus 45.91% in the sham-OVX legs (P < 0.001). These data showed that the SD rats became a suitable model of osteoporosis, 3 months after they were OVX. Immunohistochemical analysis showed higher levels of BMP-2 and OPG expression in the treatment group than those in the control group. Compared with the contralateral controls, decreased expression of OPG and BMP-2 at 3 days after roughly focused ESWT, followed by a later increase at 7 days, was indicated by real-time PCR and Western blotting analysis. The OPG messenger RNA (mRNA) expression levels peaked at 6 weeks after the shock wave treatment, paired with a much earlier (at 4 weeks) increase of BMP-2, and declined close to normal at 8 weeks.
Conclusions:
Roughly focused ESWT may promote the expression of OPG and BMP-2 in the osteoporotic fracture area in rats. BMP-2 and OPG may act synergistically and may lead to a significant enhancement of bone formation and remodeling.
Keywords: Bone Morphogenetic Protein-2, Extracorporeal Shock Waves Therapy, Fracture Healing, Osteoporotic Fracture, Osteoprotegerin
Introduction
Osteoporosis is a disease characterized by low bone mass and deterioration of the microarchitecture of the bone. Patients suffering from osteoporosis are at an increased risk for bone fractures. Fracture is the ultimate and most catastrophic consequence of osteoporosis.[1] Osteoporosis now comprises a large percentage of both public and private health spending, and these costs are expected to escalate rapidly in the 21st century. The projected costs of hip fractures alone may reach US $131 billion worldwide by 2050.[2,3,4] Fracture healing is a complex series of cellular events, the regulation of which remains poorly understood. The relationship between fracture healing and osteoporosis is also intricate.[5] Although a great deal of attention has been focused on fracture prevention and new therapies have been aimed at conserving bone mass, little emphasis has been placed on the study of fracture healing in osteoporotic bone. For patients with osteoporosis, fracture healing is delayed, recovery takes longer time, osteoporosis is aggravated, and the risk of re-fracture increases significantly after healing. The treatment of osteoporosis should involve surgery to repair osteoporotic fractures to reduce bone and so prevent re-fracture. Currently, treatment is mainly pharmacological. The necessity of lifelong treatment, the potential negative side effects, and the high costs justify the search for alternative treatments.[6] One new noninvasive treatment is extracorporeal shock waves therapy (ESWT).[7,8,9,10,11]
Extracorporeal shock wave (ESW) is a noninvasive set of acoustic waves, including electrohydraulic, electromagnetic, and piezoelectric shock waves. Electrohydraulic shock waves are high-energy shock waves (HESWs) which are used in urology for lithotripsy to disintegrate urolithiasis whereas in orthopedics, they are used to induce tissue repair and regeneration.[7,12] HESW therapy, specifically through sound waves, is produced by a reflector. The shock waves are damply conducted in line until they encounter a transmitter with the same acoustic impedance. When shock waves encounter an interface of different densities or of acoustic impedance (such as the bone tissue or calculus), their energy is released if the acoustic impedance suddenly changes during the shear stress. The cavitation effect may cause fracturing of the calculus or bone, finally causing the biochemical effect of the histiocyte, promoting bone fracture healing and bone induction. HESW can cause microcracks in the bone tissue, subperiosteal hemorrhage, microdamage to the bone trabecular, and small hemorrhage of the medullary cavity.[7,13,14] These problems can in turn cause new trauma responses of the fracture area, extend the inflammation period, stimulate large amounts of inflammation, stimulate blood vessel reactions, increase the local blood supply, induce vascularization, enhance intramembranous ossification and endochondral bone, and promote fracture healing.[15,16] It can also promote the formation of new blood vessels, promote soft tissue repair and regeneration, and relieve pain.[17,18,19,20,21,22,23]
Estrogen deficiency is a major risk factor for osteoporosis. Ovariectomized (OVX) rats are widely used as a model of postmenopausal osteoporosis. This model has been validated as a clinically relevant model of human postmenopausal bone loss.[24,25] In the present experiment, both ovaries were removed from Sprague-Dawley (SD) rats to create the model of osteoporosis. The rats were then treated with double proximal tibial osteotomy and Kirschner wire internal fixation. To investigate the effects of roughly focused ESWT on the level of osteoprotegerin (OPG) and bone morphogenetic protein-2 (BMP-2) expression in osteoporotic fractures in rats, expression of OPG and BMP-2 in the callus of the fracture area was regularly assessed using immunohistochemistry, quantitative real-time polymerase chain reaction (PCR), and Western blotting analysis.
Methods
Laboratory animals
Seventy-two female SD clean level rats, 3 months old with an average weight of 200 ± 2.5 g, were provided by Shanghai Xi Puer-Bi Kai Experimental Animal Company (China). The permit is SCXK (Shanghai) 2008-0016.
Experimental design
Model of osteoporosis
This study was approved by the related animal welfare Ethics Committee of the Sixth People's Hospital, affiliated with Shanghai Jiao Tong University.
Seventy-two female SD rats, 3 months old, were divided into sham-operated group (n = 6) and an OVX group (n = 66). Bilateral oophorectomy was performed on the rats in the OVX group. The oviduct and accompanying blood vessels were rigorously ligated, and then the incision was sutured. The bilateral ovaries of the rats in the sham-operated group were merely located, and their incisions were sutured thereafter. All postoperative rats were housed in a cage with 12 h day–night light conditions and 60% humidity at 21°C.
After conventional breeding for 3 months, 6 of the 66 rats in the OVX group were randomly selected and euthanized, and all the rats in sham-operated group were euthanized. Bone mineral density (BMD) was routinely estimated using dual-energy X-ray absorptiometry (DXA: Hologic, USA) of the L5 vertebrae, femoral neck, and proximal tibia. Osteoporotic bones in the OVX rats are here defined as bones with BMD measurements more than 2.5 SD below the mean BMD obtained from the sham-operated group.[5] At the same time, the BMD of the proximal tibia was measured using microscopic computed tomography (CT) scanning (Skyscan 1076, Belgium) to observe bone microstructure of OVX rats.
Modeling of fracture fixation
After the successful modeling of osteoporosis, an open double proximal tibial osteotomy was created and stabilized using intramedullary pins in rats from the OVX groups [Figure 1]. X-ray inspection was performed in strict accordance with the standard procedures for bilateral tibial fractures in 1/3 section transverse, and the successfully established osteoporotic fracture experimental model was selected for further research.
Figure 1.
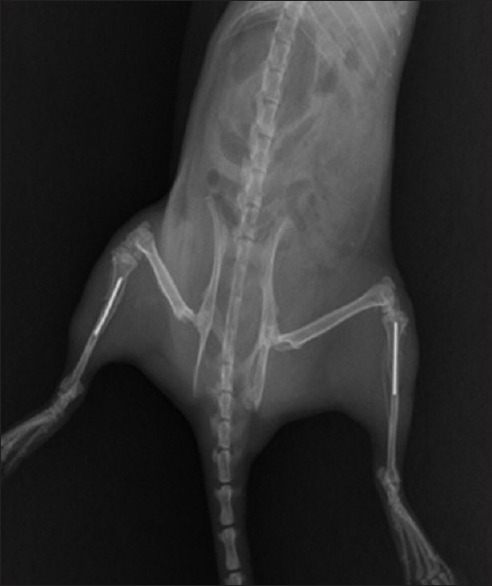
Modeling of the bilateral proximal tibial fracture fixation.
Roughly focused extracorporeal shock waves therapy
After internal fixation for 1 week, the center of the site of the fracture of the left tibia was marked with methyl violet and treated once with roughly focused ESWT at an energy density of 0.26 mJ/mm2, 60 doses each minute and a rate of recurrence, and 2000 pact quantities to the left tibia using Orthospec™ (Medispec, Israel). The contralateral, right tibia was not treated and served as a control. After treatment, the rats were conventionally bred. Expression of OPG and BMP-2 in the callus of the osteoporotic fracture area was assessed using immunohistochemistry, real-time PCR, and Western blotting analysis.
Testing
Immunohistochemistry
Six SD rats were selected at random and euthanized at 2, 4, and 8 weeks after treated with roughly focused ESWT. Then, 1 cm bone tissue was intercepted at the midpoint of the tibial fracture. Then, the tibial specimens were fixed with 10% formalin, decalcified with 5% ethylenediaminetetraacetic acid, conventionally dehydrated and degreased, embedded with low-melting point paraffin (56°C), and cut into 7 μm serial sections using a hard tissue slice machine (LEICA SP1600 and SW2500, Germany). The slices were stained using immunohistochemistry with chain mold avidin marked with peroxidase (streptavidin-peroxidase, S-P). The valence of BMP-2 and protection element (OPG) monoclonal antibody was 1:100. The slices were observed under a microscope (Leica DM4000B, Germany).
Real-time quantitative polymerase chain reaction
Six SD rats were selected at random and sacrificed at 1st and 3rd days, 1, 2, 4, 6, and 8 weeks after treated with roughly focused ESWT. Then, 1 cm callus tissue was intercepted at the midpoint of the tibial fracture. The samples were quick frozen in liquid nitrogen and stored at −80°C for later real-time PCR and Western blotting analysis. Total RNA was extracted using Trizol reagent (Life Technologies, Grand Island, NY, USA) and precipitated in ethanol. Purity and concentration were assessed by agarose gel electrophoresis and ultraviolet spectrophotometry.
Reverse transcription followed by quantitative real-time PCR was performed as described previously. TaqMan primer and probe pairs for the rat OPG, BMP-2 gene, and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an internal control were based on database sequences of Genbank and were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA). The primer sequences were as follows: rat BMP-2, forward: 5’ ACGACGGTAAAGGACATC 3’; reverse: 5’ ATGGTTGGTGGAGTTCAG 3’; rat OPG, forward: 5’ CTGGGCTGTTTCTTCAGGATG 3’; reverse: 5’ CTCTTTCTCAGGGTGCTTGAC 3’; rat GAPDH, forward: 5’ AAACTCACTGGCATGGCCTT 3’; and reverse: 5’ TTAGCAGCTTTCTCCAGGCG 3’. Real-time quantitative PCR was done in triplicate with the ABI 7300 Sequence Detector (Applied Biosystems) following the recommended protocols. Results were normalized to GAPDH levels using the following formula: threshold cycle (Ct) = Ct of target gene − Ct of GAPDH. The comparative Ct method was used to investigate the amount of target gene relative to a calibrator. Of the 42 animals, an arbitrary animal was selected as calibrator. The ΔΔCt value was calculated as follows: ΔΔCt of an animal = ΔCt of an animal − ΔCt of the calibrator. The amount of target gene normalized to GAPDH and relative to a calibrator of an animal was given by the formula 2−ΔΔCt. Then, the amount of mRNA expression was compared between the shock wave-treated osteoporotic fracture and the untreated controls. The fold change between the shock wave-treated osteoporotic fracture and untreated control was calculated as follows: value of the shock wave-treated osteoporotic fracture/value of the untreated control, at each time point.
Western blotting analysis
For Western blotting, proteins were extracted from the tibial callus tissue samples in a radioimmunoprecipitation buffer (eight times the sample volume) containing a Protease Inhibitor Cocktail (Protease Inhibitor Cocktail Set I; Calbiochem, Darmstadt, Germany) at 4°C overnight. The proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (10% gels). A human recombinant OPG, BMP-2 (Abcam, Cambridge, UK), and Marker Prestained Protein Standard (Fermentas) were also loaded as positive control and as molecular weight markers, respectively. Proteins were transferred to a polyvinylidene difluoride membrane (Invitrogen), blocked with 2% bovine serum albumin (BSA), and exposed to a 1:100 dilution of the mouse antibody used for immunohistochemistry (R&D Systems, Minneapolis, MN, USA) in 1% BSA overnight. After washing in 0.1% Tween in PBS, the membrane was incubated in goat anti-mouse IgG horseradish peroxidase-linked antibody (1:5000) for 1 h at room temperature and visualized using a nonradioactive ECL kit (Amersham Biosciences, Piscataway, NJ, USA). GAPDH Western blots were performed on the same membrane after a stripping procedure. Samples from each animal were repeated twice, and the results were found to be the same.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Statistical software used herein was SAS 9.1 (SAS Institute Inc., USA). Means between groups were compared using t-test and rates were compared using Chi-square test. P values were obtained using the Student's t-test. P < 0.05 was considered statistically significant.
Results
Effects of bone mineral density and bone microstructure of ovariectomized rats
BMD at the proximal tibia, l femur, and L5 spine was significantly lower in the OVX group than that in sham-operated rats [Table 1], but the total vertebral areas were similar for both groups (data not shown). Micro-CT scanning showed bone microstructure of the sham-operated group rats to be obviously superior to that of the OVX group [Figure 2].
Table 1.
Comparison of absolute bone mineral density DXA values in sham-operated and OVX rats (g/cm2)
| Groups | n | Proximal tibia | L5 | Proximal femur |
|---|---|---|---|---|
| Sham-operated group | 6 | 0.928 ± 0.072 | 0.271 ± 0.006 | 0.452 ± 0.021 |
| OVX group | 6 | 0.808 ± 0.090 | 0.236 ± 0.005 | 0.356 ± 0.017 |
| t | 2.550 | 10.977 | 8.703 | |
| P | <0.05 | <0.001 | <0.001 | |
Measurements were performed at various sites, including proximal tibia, proximal femur, and L5. Absolute bone mineral density values expressed as mean (g/cm2) ±95% CI. The P values were obtained using Student’s t-test. CI: Confidence interval; OVX: Ovariectomized; DXA: Dual-energy X-ray absorptiometry.
Figure 2.
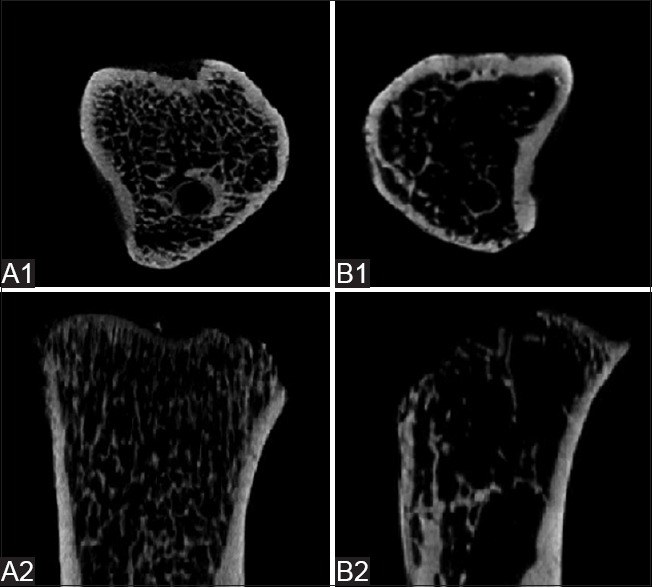
Images of micro-CT scanning of proximal tibias in the (A1 and A2) sham-operated and (B1 and B2) OVX rats. Micro-CT scanning showed bone microstructure of the sham-operated group rats to be obviously superior to that of the OVX group. CT: Computed tomography; OVX: Ovariectomized.
In addition, Micro-CT detection data showed that at the proximal tibias, the lower trabecular bone volume fraction (BV/TV), the mean trabecular thickness (Tb. Th), and the mean trabecular number (Tb.N) of the treatment group were significantly higher than that of the control group. The mean structure model index (SMI) and trabecular separation (Tb. Sp) were lower in the sham-operated group than in the OVX group. The difference was found to be statistically significant (P < 0.05) [Table 2].
Table 2.
Space parameters of the proximal tibia specimens in sham-operated and ovariectomized rats (mean ± SD)
| Groups | n | BV/TV (%) | SMI | Trabecular thickness (mm) | Trabecular number (mm) | Trabecular separation (mm) |
|---|---|---|---|---|---|---|
| Sham-operated group | 6 | 45.91 ± 4.87 | 0.62 ± 0.12 | 0.18 ± 0.06 | 2.75 ± 0.49 | 0.23 ± 0.07 |
| OVX group | 6 | 14.29 ± 3.38 | 2.31 ± 0.46 | 0.11 ± 0.04 | 0.97 ± 0.26 | 0.53 ± 0.12 |
| t | 13.055 | 8.71 | 2.38 | 7.86 | 5.29 | |
| P | 0.000 | <0.001 | <0.05 | <0.001 | <0.001 | |
OVX: Ovariectomized; SD: Standard deviation; SMI: Structure model index; BV/TV: Lower trabecular bone volume fraction.
Immunohistochemistry
BMP-2 and OPG-positive staining appeared brown. It was observed that the reaction of OPG and BMP-2 in the treatment group was strongly positive at 2 and 4 weeks after treated with roughly focused ESWT relative to the control group. At 8 weeks, the reconstruction of the trabecular bone was obviously superior in the treatment group. BMP-2 and OPG were mainly distributed in the bone marrow tissue around fracture of the medullary cavity, especially in the active site of bone formation and bone resorption [Figures 3 and 4].
Figure 3.
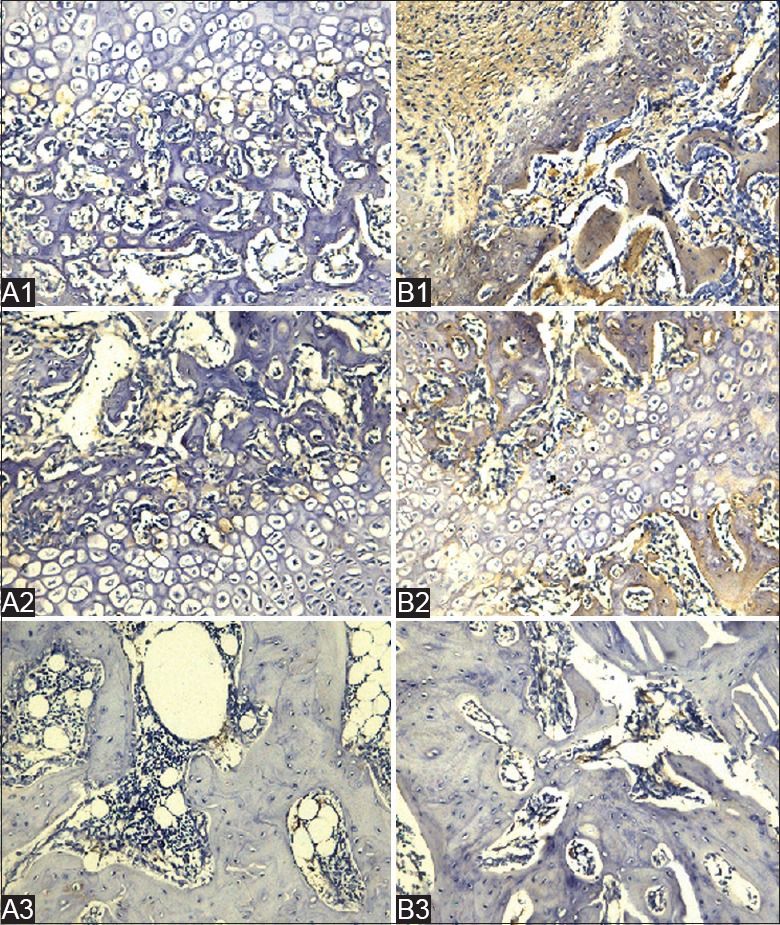
A1, A2, and A3 show control groups at 2, 4, and 8 weeks, respectively. B1, B2, and B3 show treatment groups at 2, 4, and 8 weeks, respectively, after shock wave treatment. B1 shows more collagen (fibroblasts), osteoblasts, and ossification than A1. In the control group, BMP-2 was mainly expressed in fibroblasts, less collagen formed, and there were fewer osteoblasts. The reaction of BMP-2 in the treatment group was strongly positive. As shown in A2 and B2, at 4 weeks after treatment, BMP-2 was mainly expressed in hypertrophy cartilage cells and the osteoblasts of newly formed woven bone. As shown in A3 and B3, the trabeculae of treatment group had finished forming lamellar bone, and the fiber callus had disappeared. The reconstruction of the trabecular bone of control group was not completed, and BMP-2 remained visible in the marrow cavity (immunohistochemical staining, Original magnification ×200: SP method). BMP: Bone morphogenetic protein-2; SP: Streptavidin - peroxidase.
Figure 4.
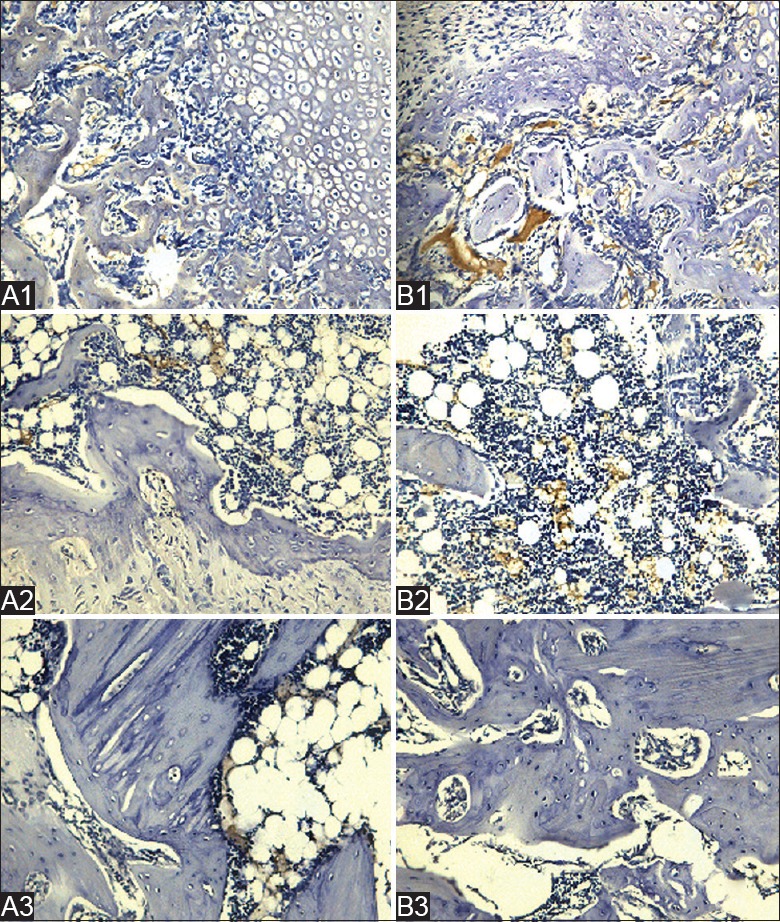
A1, A2, and A3 show control groups at 2, 4, and 8 weeks, respectively. B1, B2, and B3 show treatment groups at 2, 4, and 8 weeks, respectively, after shock wave treatment. B1 shows more plentiful collagen, osteoblasts, and ossification than A1. OPG levels were shown to increase in response to the treatment. OPG was mainly expressed in myeloid tissues of the marrow cavity at 4 weeks after the treatment, as shown in A2 and B2. B3 shows that the reconstruction of the trabecular bone had basically been completed and fiber callus disappeared at 8 weeks. The reparative process was not adequately completed, and OPG remained visible in the marrow cavity (immunohistochemical staining, Original magnification ×200: SP method). OPG: Osteoprotegerin; SP: Streptavidin - peroxidase.
Real-time polymerase chain reaction
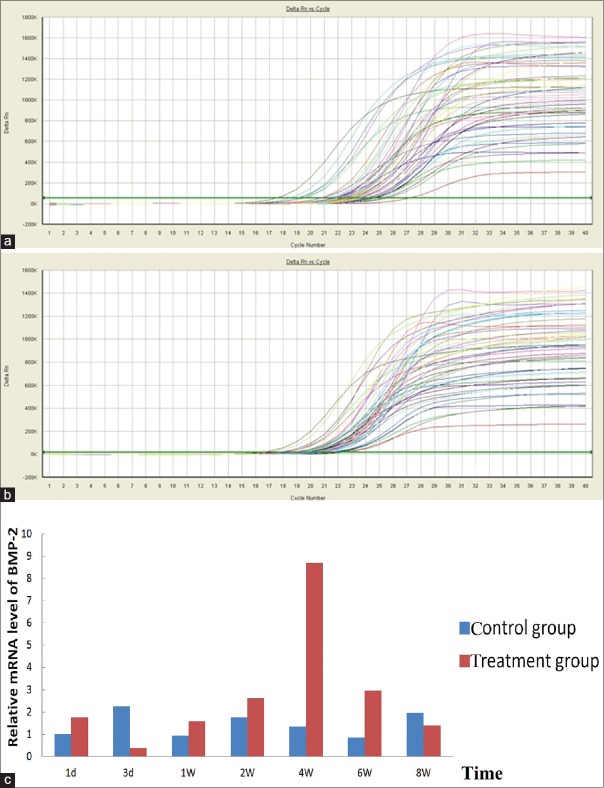
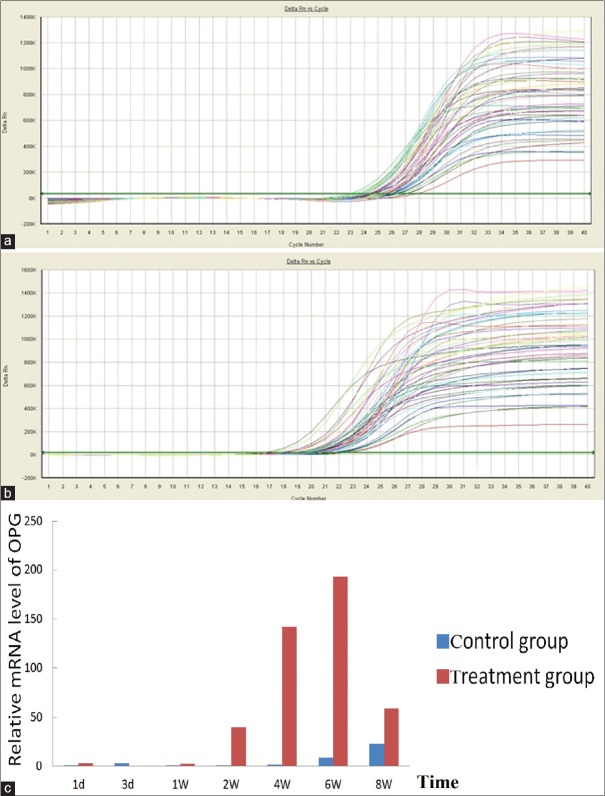
The rats were treated with roughly focused ESWT once by energy density of 0.26 mJ/mm2, 60 each minute of rate of recurrence, and 2000 pact quantities to the left tibia, and real-time PCR showed that the shock wave therapy significantly increased OPG and BMP-2 mRNA expression in the callus in the osteoporotic fracture area of rats. Treated rats showed less expression of OPG and BMP-2, 3 days after treated with roughly focused ESWT than control rats. This was followed by a later increase of OPG at 7 days, as indicated by the results of real-time PCR. The OPG mRNA expression levels peaked 6 weeks after the shock wave treatment, paired with a much earlier increase of BMP-2 at 4 weeks, and dropped close to normal at 8 weeks [Figures 5 and 6].
Figure 5.
(a) Amplification of kinetic curves of BMP-2 mRNA expression; (b) amplification of kinetic curves of GAPDH mRNA expression; (c) comparison of BMP-2 mRNA expression in the control to that of the treatment group at 1, 3 days, 1, 2, 4, 6, and 8 weeks after treated with roughly focused ESWT. BMP-2 mRNA expression in the treatment group and in the control group, P < 0.05 (data not shown) (note: BMP-2 mRNA expression at 1 day after roughly focused ESWT was normalized to 1). ESWT: Extracorporeal shock waves therapy; BMP-2: Bone morphogenetic protein-2; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; mRNA: Messenger RNA.
Figure 6.
(a) Amplification of kinetic curves of OPG mRNA expression; (b) amplification of kinetic curves of GAPDH mRNA expression; (c) comparison of OPG mRNA expression in the control to that of the treatment group at 1, 3 days, 1, 2, 4, 6, and 8 weeks after roughly focused ESWT. OPG mRNA expression in the treatment group and in the control group, P < 0.05 (data not shown) (note: BMP-2 mRNA expression at 1 day after roughly focused ESWT was normalized to 1). BMP-2: Bone morphogenetic protein-2; OPG: Osteoprotegerin; mRNA: Messenger RNA; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; ESWT: Extracorporeal shock waves therapy.
Osteoprotegerin and bone morphogenetic protein-2 protein expression
An increase in both density and broadness of the band was consistently observed in the healing tibia samples obtained from the shock wave-treated osteoporotic tibia fractures at 7 days, 2, 4, and 6 weeks after treated with roughly focused ESWT, compared with the samples from the untreated tibia. However, treated tibia showed less protein expression of OPG and BMP-2 at 3 days after the treatment than control rats. In the samples obtained 1 day and 8 weeks after treated with roughly focused ESWT, there was no difference between the results from the treated osteoporotic tibia fractures and the untreated osteoporotic tibia fractures [Figure 7].
Figure 7.
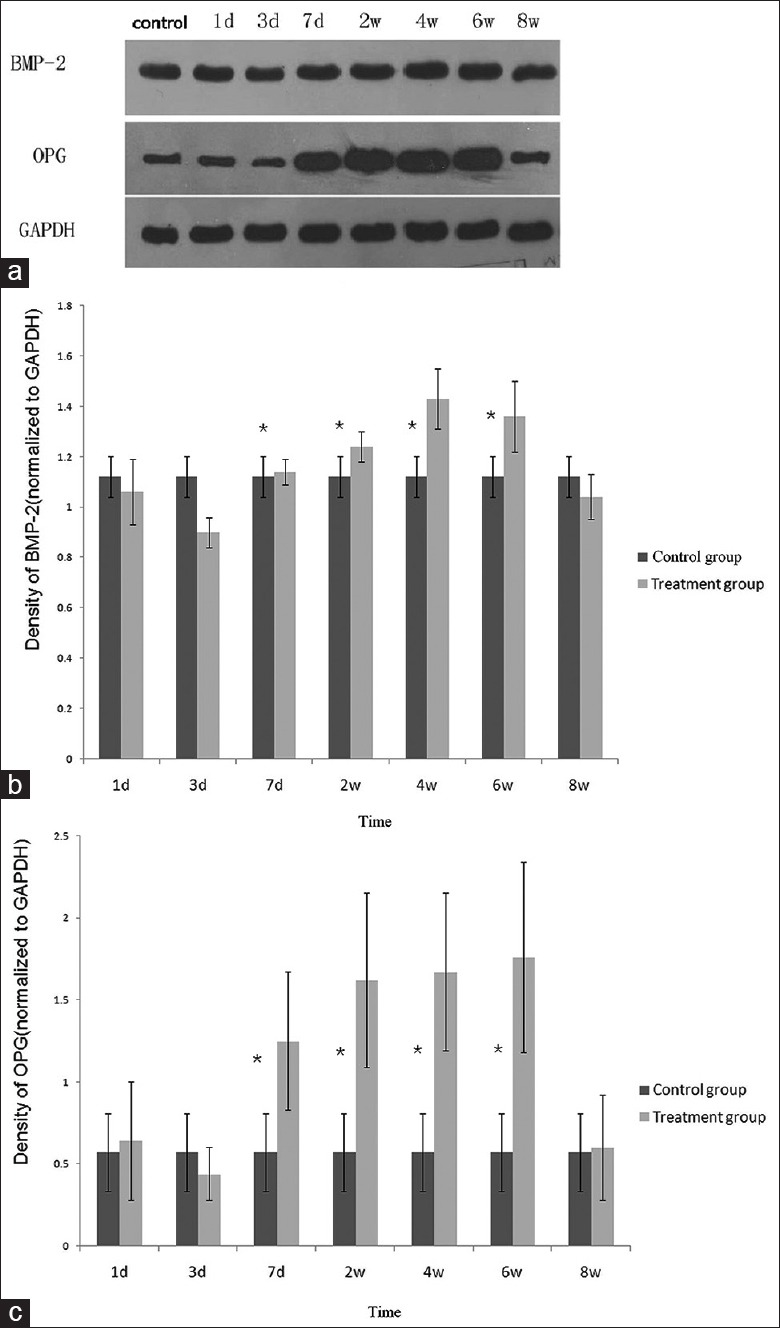
(a) Western blotting analysis of the callus tissue samples obtained from tibial fracture in rats at 1st and 3rd days, 1, 2, 4, 6, and 8 weeks after the application of shock wave treatment (Lanes 1st and 3rd days, 1, 2, 4, 6, and 8 weeks represent 1, 3, and 7 days and 2, 4, 6, and 8 weeks, respectively). Western blotting analysis consistently showed increased BMP-2 and OPG expression at 1, 2, 4, and 6 weeks compared to the samples from the untreated control tibial fracture. (b and c) Graph showing densitometric analysis of Western blotting about BMP-2 and OPG expression, respectively, normalized to the density of GAPDH in each lane. The asterisks indicate statistically significant differences between shock wave-treated tibias and untreated tibias at each time point. BMP-2: Bone morphogenetic protein-2; OPG: Osteoprotegerin; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Discussion
This study has shown that the influence of bone mass and bone microarchitecture on the early phase in a rat osteoporotic model can be induced by ovx.[26] BMDs at the proximal tibia, femur, and L5 spine were significantly reduced after ovariectomy. BMD of proximal tibia was 12.9% less in the OVX group than in the sham-operated group, indicating that the proximal tibia was sensitive to OVX-induced bone loss [Table 1]. A bilateral oophorectomy produced a higher BV/TV, Tb. Th, and Tb.N in the proximal tibia of the sham-OVX animals than in other animals. Tb. Sp and SMI were significantly lower in the OVX group than in the sham-operated group. These data showed that bone microarchitecture can be affected after ovariectomy.
Three months after bilateral oophorectomy, the changes in BMD and bone microarchitecture in the OVX rats confirmed osteoporosis. These data confirmed the value of our model in evaluating the healing process of fractured bone under osteoporotic conditions. This model may be of benefit for testing the effects of new therapies meant to promote healing of osteoporotic fractures.[5]
Estrogen deficiency is a major cause of postmenopausal osteoporosis.[27] Estrogen promoted OPG expression[28] in a dose- and time-dependent manner.[28] OPG plays an important role in the negative regulation of osteoclastic bone resorption.[28] In this way, estrogen enhancement of OPG secretion by osteoblastic cells may play a major role in the antiresorptive action of estrogen on bone. In the present study, osteoporotic rats were treated once with roughly focused ESWT by energy density of 0.26 mJ/mm2, 60 doses/min, and 2000 pact quantities to the left tibia. Immunohistochemical detection showed compared with the control group, plentiful collagen, osteoblasts, and ossification in the treatment group were more; OPG levels were shown to increase in response to the treatment. OPG increased by the shock waves therapy prevents bone loss and induces osteoporosis when administered to OVX rats.[28] Two weeks after injection of OPG (5 mg·kg−1·d−1) or the administration of OPG-encoding recombinant adenovirus, the bone quantity of spayed osteoporosis mice increased significantly. There were no abnormal changes observed in other tissues or organs.[29,30]
BMPs are hydrophobic acid polypeptides capable of inducing the proliferation and differentiation of original interstitial cells to bone and cartilage cells.[31] The ability of BMPs to induce osteogenesis was confirmed by Ren et al.[32] Among twenty subtypes of BMP, BMP-2 was found to have the most pronounced ability to induce ossification.[33] In this study, compared with the control group, BMP-2 levels were shown to increase in response to the treatment in the osteoporotic fractured area; BMP-2 was mainly expressed in fibroblasts, less collagen formed, and there were fewer osteoblasts. The results of immunohistochemistry in the current study support the findings of some experiments which showed that ESW treatment significantly upregulated the expression of BMP-2 in some musculoskeletal diseases.[34,35,36,37] Ras protein and BMP increased by the shock waves treatment were found to induce the differentiation of undifferentiated mesenchymal cell into cells capable of osteogenesis and promote the formation of bone and cartilage.[38] Meanwhile, the results of the present study showed roughly focused ESWT to be effective in the treatment of osteoporotic fractures by inhibiting bone resorption and increasing bone formation by enhancing OPG and BMP-2.
ESWT has been shown to increase the expression of several growth factors.[6] BMP-2 and OPG may act collectively and lead to noteworthy enhancement of bone formation and remodeling.[39] In the present study, real-time PCR and Western blotting analysis showed that the shock waves therapy significantly increase the expression of BMP-2 and OPG in the callus of the osteoporotic fracture area of rats at all times, including 1, 7 days, 2, 4, 6, and 8 weeks, except on day 3. In other word, real-time PCR and Western blotting analysis also proved that the roughly focused ESWT significantly upregulated the expression of BMP-2 and OPG in osteoporotic tibia fractures area in rats. Many members of BMPs can stimulate OPG mRNA expression.[40] A 100 ng/ml dose of BMP-2 was found to increase the concentration of OPG mRNA by 400% and the concentration of OPG protein by 80% in human embryonic osteoblasts.[41] All kinds of local growth factors, such as tumor necrosis factor (TNF)-α, TNF-β, and BMP-2, adjust the differentiation of osteoclast precursor cells and the activity of mature osteoclasts through the regulation of OPG/receptor activator of nuclear factor κB ligand (RANKL) ratio.[42,43] Recent studies have shown that BMP-2 markedly enhanced osteoclast differentiation induced by RANKL and macrophage colony-stimulating factor.[41] The addition of BMP receptor type IA to the culture can significantly inhibit osteoclast formation induced by RANKL and BMP-2. BMP-2 increased the survival of purified osteoclasts supported by RANKL.[44] In addition, BMP-2 promoted the synthesis of Smad 1, which can bind to the two Hoxc-8 sites on the OPG promoter, resulting in the increase of OPG secretion in osteoblast cells.[40] These findings suggest that BMP-mediated signals cross communicate with RANKL-mediated signals and so induce osteoclast differentiation and survival.[44]
In conclusion, the SD rats became a suitable model of osteoporosis 3 months after they were OVX. Roughly focused ESWT has beneficial effects on the osteoporotic fracture healing in rats. It may promote the expression of OPG and BMP-2 in the osteoporotic fracture area in rats. BMP-2 and OPG may act synergistically and may lead to significant enhancement of bone formation and remodeling.
Financial support and sponsorship
This work was supported by grants from the National Nature Science Foundation of China (No. 81071501) and the Shanghai Committee of Science and Technology, China (No. 09411966500).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
References
- 1.Consensus development conference: Prophylaxis and treatment of osteoporosis. Am J Med. 1991;90:107–10. doi: 10.1016/0002-9343(91)90512-v. doi: 0002-9343(91)90512-V[pii] [DOI] [PubMed] [Google Scholar]
- 2.Johnell O. The socioeconomic burden of fractures: Today and in the 21st century. Am J Med. 1997;103:20S–5S. doi: 10.1016/s0002-9343(97)90023-1. doi: 10.1016/S0002-9343(97)90023-1. [DOI] [PubMed] [Google Scholar]
- 3.Chrischilles E, Shireman T, Wallace R. Costs and health effects of osteoporotic fractures. Bone. 1994;15:377–86. doi: 10.1016/8756-3282(94)90813-3. doi: 10.1016/8756-3282(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 4.Jones G, Nguyen T, Sambrook PN, Kelly PJ, Gilbert C, Eisman JA. Symptomatic fracture incidence in elderly men and women: The Dubbo Osteoporosis Epidemiology Study (DOES) Osteoporos Int. 1994;4:277–82. doi: 10.1007/BF01623352. doi: 10.1007/BF01623352. [DOI] [PubMed] [Google Scholar]
- 5.Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, et al. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–6. doi: 10.1016/s8756-3282(00)00414-2. doi: 10.1016/S8756-3282(00)00414-2. [DOI] [PubMed] [Google Scholar]
- 6.van der Jagt OP, van der Linden JC, Schaden W, van Schie HT, Piscaer TM, Verhaar JA, et al. Unfocused extracorporeal shock wave therapy as potential treatment for osteoporosis. J Orthop Res. 2009;27:1528–33. doi: 10.1002/jor.20910. doi: 10.1002/jor.20910. [DOI] [PubMed] [Google Scholar]
- 7.Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11. doi: 10.1186/1749-799X-7-11. doi: 10.1186/1749-799X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CJ, Yang KD, Wang FS, Hsu CC, Chen HH. Shock wave treatment shows dose-dependent enhancement of bone mass and bone strength after fracture of the femur. Bone. 2004;34:225–30. doi: 10.1016/j.bone.2003.08.005. doi: 10.1016/j.bone.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Valchanou VD, Michailov P. High energy shock waves in the treatment of delayed and nonunion of fractures. Int Orthop. 1991;15:181–4. doi: 10.1007/BF00192289. doi: 10.1007/BF00192289. [DOI] [PubMed] [Google Scholar]
- 10.Furia JP, Rompe JD, Cacchio A, Maffulli N. Shock wave therapy as a treatment of nonunions, avascular necrosis, and delayed healing of stress fractures. Foot Ankle Clin. 2010;15:651–62. doi: 10.1016/j.fcl.2010.07.002. doi: 10.1016/j.fcl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Rompe JD, Theis C, Maffulli N. Shock wave treatment for tennis elbow. Orthopade. 2005;34:567–70. doi: 10.1007/s00132-005-0805-x. doi: 10.1007/s00132-005-0805-x. [DOI] [PubMed] [Google Scholar]
- 12.Ogden JA, Tóth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res. 2001;387:8–17. doi: 10.1097/00003086-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Chen XF, Huang HM, Li XL, Liu GJ, Zhang H. Slightly focused high-energy shockwave therapy: A potential adjuvant treatment for osteoporotic fracture. Int J Clin Exp Med. 2015;8:5044–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Dorotka R, Kubista B, Schatz KD, Trieb K. Effects of extracorporeal shock waves on human articular chondrocytes and ovine bone marrow stromal cells in vitro. Arch Orthop Trauma Surg. 2003;123:345–8. doi: 10.1007/s00402-003-0551-7. doi: 10.1007/s00402-003-0551-7. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WT, Preminger GM. Extracorporeal shock wave lithotripsy. An update. Urol Clin North Am. 1990;17:231–42. [PubMed] [Google Scholar]
- 16.Rodolà F, Conti C, Abballe C, Chierichini A, Ciano F, Forte E, et al. Anaesthesia for shock wave therapy in musculoskeletal disorders: A preliminary report. Eur Rev Med Pharmacol Sci. 2002;6:133–8. [PubMed] [Google Scholar]
- 17.Wang CJ, Huang KE, Sun YC, Yang YJ, Ko JY, Weng LH, et al. VEGF modulates angiogenesis and osteogenesis in shockwave-promoted fracture healing in rabbits. J Surg Res. 2011;171:114–9. doi: 10.1016/j.jss.2010.01.045. doi: 10.1016/j.jss.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Wang FS, Yang KD, Chen RF, Wang CJ, Sheen-Chen SM. Extracorporeal shock wave promotes growth and differentiation of bone-marrow stromal cells towards osteoprogenitors associated with induction of TGF-beta1. J Bone Joint Surg Br. 2002;84:457–61. doi: 10.1302/0301-620x.84b3.11609. doi: 10.1302/0301-620X.84B3.11609. [DOI] [PubMed] [Google Scholar]
- 19.Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, Huang HC, et al. Recruitment of mesenchymal stem cells and expression of TGF-beta 1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526–34. doi: 10.1016/j.orthres.2003.10.005. doi: 10.1016/j.orthres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Reichenberger MA, Heimer S, Schaefer A, Lass U, Gebhard MM, Germann G, et al. Extracorporeal shock wave treatment protects skin flaps against ischemia-reperfusion injury. Injury. 2012;43:374–80. doi: 10.1016/j.injury.2011.11.019. doi: 10.1016/j.injury.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi AA, Ross KM, Ogawa R, Orgill DP. Shock wave therapy in wound healing. Plast Reconstr Surg. 2011;128:721e–7e. doi: 10.1097/PRS.0b013e318230c7d1. doi: 10.1097/PRS.0b013e318230c7d1. [DOI] [PubMed] [Google Scholar]
- 22.Knobloch K, Vogt PM. High-energy focussed extracorporeal shockwave therapy reduces pain in plantar fibromatosis (Ledderhose's disease) BMC Res Notes. 2012;5:542. doi: 10.1186/1756-0500-5-542. doi: 10.1186/1756-0500-5-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariotto S, de Prati AC, Cavalieri E, Amelio E, Marlinghaus E, Suzuki H. Extracorporeal shock wave therapy in inflammatory diseases: Molecular mechanism that triggers anti-inflammatory action. Curr Med Chem. 2009;16:2366–72. doi: 10.2174/092986709788682119. [DOI] [PubMed] [Google Scholar]
- 24.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–91. doi: 10.1016/0169-6009(91)90124-i. doi: 10.1016/0169-6009(91)90124-I. [DOI] [PubMed] [Google Scholar]
- 25.Wronski TJ, Dann LM, Qi H, Yen CF. Skeletal effects of withdrawal of estrogen and diphosphonate treatment in ovariectomized rats. Calcif Tissue Int. 1993;53:210–6. doi: 10.1007/BF01321840. doi: 10.1007/BF01321840. [DOI] [PubMed] [Google Scholar]
- 26.Wronski TJ, Lowry PL, Walsh CC, Ignaszewski LA. Skeletal alterations in ovariectomized rats. Calcif Tissue Int. 1985;37:324–8. doi: 10.1007/BF02554882. doi: 10.1007/BF02554882. [DOI] [PubMed] [Google Scholar]
- 27.Das S, Crockett JC. Osteoporosis – A current view of pharmacological prevention and treatment. Drug Des Devel Ther. 2013;7:435–48. doi: 10.2147/DDDT.S31504. doi: 10.2147/dddt.s31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–70. doi: 10.1210/endo.140.9.7131. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 29.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 30.Bolon B, Carter C, Daris M, Morony S, Capparelli C, Hsieh A, et al. Adenoviral delivery of osteoprotegerin ameliorates bone resorption in a mouse ovariectomy model of osteoporosis. Mol Ther. 2001;3:197–205. doi: 10.1006/mthe.2001.0245. doi: 10.1006/mthe.2001.0245. [DOI] [PubMed] [Google Scholar]
- 31.Alden TD, Varady P, Kallmes DF, Jane JA, Jr, Helm GA. Bone morphogenetic protein gene therapy. Spine (Phila Pa 1976) 2002;27(16 Suppl 1):S87–93. doi: 10.1097/00007632-200208151-00016. [DOI] [PubMed] [Google Scholar]
- 32.Ren XY, Ruan QR, Zhu DH, Zhu M, Qu ZL, Lu J. Tetramethylpyrazine inhibits agiontensin II-induced nuclear factor-kappaB activation and bone morphogenetic protein-2 downregulation in rat vascular smooth muscle cells. Acta physiol Sin. 2007;59:339–44. [PubMed] [Google Scholar]
- 33.Reddi AH. Initiation of fracture repair by bone morphogenetic proteins. Clin Orthop Relat Res. 1998;355(Suppl):S66–72. doi: 10.1097/00003086-199810001-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lee TC, Wang CJ, Yang YL, Huang YH, Lin WC, Chang SY. Bone morphogenetic protein-2 expression in spinal fusion masses enhanced by extracorporeal shock wave treatment: A rabbit experiment. Acta Neurochir (Wien) 2010;152:1779–84. doi: 10.1007/s00701-010-0744-0. doi: 10.1007/s00701-010-0744-0. [DOI] [PubMed] [Google Scholar]
- 35.Baltzer AW, Ostapczuk MS, Stosch D, Granrath M. The use of recombinant human bone morphogenetic protein-2 for the treatment of a delayed union following femoral neck open-wedge osteotomy. Orthop Rev (Pavia) 2012;4:e4. doi: 10.4081/or.2012.e4. doi: 10.4081/or.2012.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma HZ, Zeng BF, Li XL, Chai YM. Temporal and spatial expression of BMP-2 in sub-chondral bone of necrotic femoral heads in rabbits by use of extracorporeal shock waves. Acta Orthop. 2008;79:98–105. doi: 10.1080/17453670710014833. doi: 10.1080/17453670710014833. [DOI] [PubMed] [Google Scholar]
- 37.Chen YJ, Kuo YR, Yang KD, Wang CJ, Huang HC, Wang FS. Shock wave application enhances pertussis toxin protein-sensitive bone formation of segmental femoral defect in rats. J Bone Miner Res. 2003;18:2169–79. doi: 10.1359/jbmr.2003.18.12.2169. doi: 10.1359/jbmr.2003.18.12.2169. [DOI] [PubMed] [Google Scholar]
- 38.Wang FS, Wang CJ, Huang HJ, Chung H, Chen RF, Yang KD. Physical shock wave mediates membrane hyperpolarization and Ras activation for osteogenesis in human bone marrow stromal cells. Biochem Biophys Res Commun. 2001;287:648–55. doi: 10.1006/bbrc.2001.5654. doi: 10.1006/bbrc.2001.5654. [DOI] [PubMed] [Google Scholar]
- 39.Yao Y, Huang H, Chang S, Wang C, Wang G. The effect of bone morphogenetic protein-2 and osteoprotegerin in trans-sutural distraction osteogenesis. West Chin J Stomatol. 2012;30:425–9. [PubMed] [Google Scholar]
- 40.Wan M, Shi X, Feng X, Cao X. Transcriptional mechanisms of bone morphogenetic protein-induced osteoprotegrin gene expression. J Biol Chem. 2001;276:10119–25. doi: 10.1074/jbc.M006918200. doi: 10.1074/jbc.M006918200. [DOI] [PubMed] [Google Scholar]
- 41.Fuller K, Lean JM, Bayley KE, Wani MR, Chambers TJ. A role for TGFbeta(1) in osteoclast differentiation and survival. J Cell Sci. 2000;113(Pt 13):2445–53. doi: 10.1242/jcs.113.13.2445. [DOI] [PubMed] [Google Scholar]
- 42.Brändström H, Jonsson KB, Vidal O, Ljunghall S, Ohlsson C, Ljunggren O. Tumor necrosis factor-alpha and -beta upregulate the levels of osteoprotegerin mRNA in human osteosarcoma MG-63 cells. Biochem Biophys Res Commun. 1998;248:454–7. doi: 10.1006/bbrc.1998.8993. doi: 10.1006/bbrc.1998.8993. [DOI] [PubMed] [Google Scholar]
- 43.Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by Vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun. 1998;250:776–81. doi: 10.1006/bbrc.1998.9394. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]
- 44.Itoh K, Udagawa N, Katagiri T, Iemura S, Ueno N, Yasuda H, et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142:3656–62. doi: 10.1210/endo.142.8.8300. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]