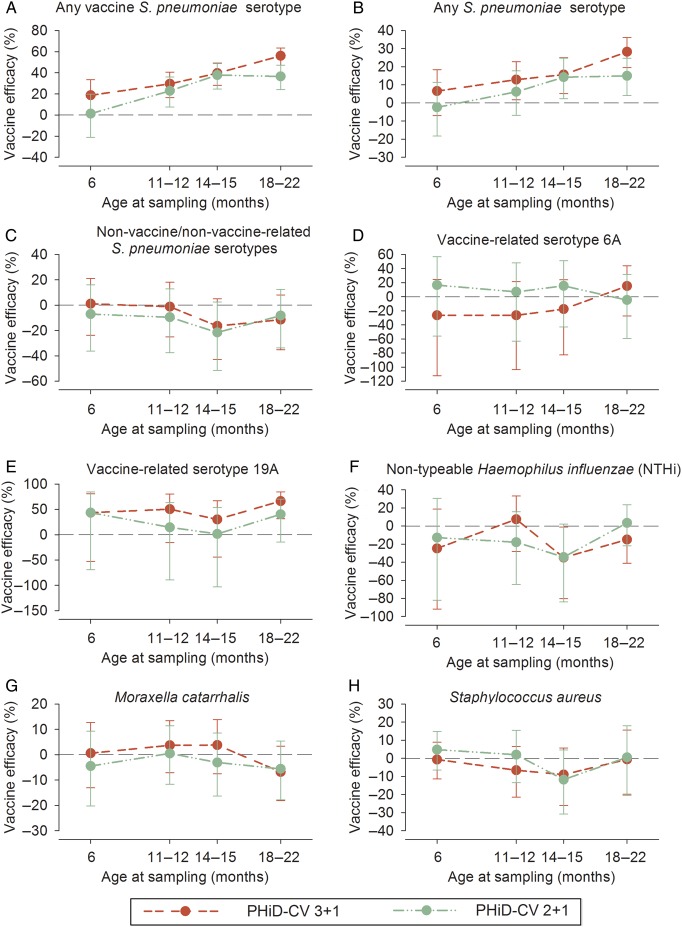
Figure 5.
Vaccine effectiveness against nasopharyngeal carriage at given time points (infant total vaccinated cohort for carriage). Vaccine efficacy against nasopharyngeal carriage of S pneumoniae, NTHi, M catarrhalis, and S aureus was assessed at different ages: 6 months (1 month after primary vaccination), 11–12 months (before booster), 14–15 months (3 months after booster), and 18–22 months (7–12 months after booster). Mean values with 95% confidence intervals are shown. Vaccine-type S pneumoniae serotypes were 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F; non-vaccine/non–vaccine-related serotypes were any S pneumoniae serotype, excluding the vaccine serotypes and any serotype that belonged to the same serogroup as the vaccine serotype. Abbreviation: PHiD-CV, 10-valent pneumococcal polysaccharide nontypeable Haemophilus influenzae protein D–conjugated vaccine.