Abstract
Idiopathic intracranial hypertension (IIH) is a disorder of elevated intracranial pressure of unknown cause occurring predominantly in young women of childbearing age. The typical patient symptom profile is the presence of daily headache, pulse synchronous tinnitus, transient visual obscurations and papilledema with its associated visual loss. The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT), is a multicenter, double-blind, randomized, placebo-controlled study of weight-reduction and low sodium diet plus acetazolamide vs diet plus placebo in subjects with mild visual loss. The study found statistically significant improvements in visual field function, quality of life measures, papilledema grade and CSF pressure in the acetazolamide group. While surgical procedures are performed for those that fail medical therapy, their relative efficacy remains unclear. The main morbidity of IIH is from visual loss. This is present in most patients and can usually be reversed if recognized early in the patient’s course and treated.
Keywords: idiopathic intracranial hypertension, pseudotumor cerebri, acetazolamide, optical coherence tomography
Idiopathic intracranial hypertension (IIH) is a disorder of overweight women in the childbearing age characterized by increased intracranial pressure with its associated signs and symptoms in an alert and oriented patient. Neuroimaging and cerebrospinal fluid (CSF) analysis is normal except for raised intracranial pressure and its associated symptoms and signs. Also, no secondary cause of intracranial hypertension is apparent. This set of criteria comprises the modified Dandy criteria (Table 1). In the last three years, there has been an explosion of publications on idiopathic intracranial hypertension with a PubMed search revealing over 500 articles. In this review, we will discuss the most important of these articles, especially those generated from the Idiopathic Intracranial Hypertension Treatment Trial (IIHT).
Table 1.
The modified Dandy criteria for IIH used in the IIHTT.
|
Prior to three years ago, a Cochrane review concluded, “There is insufficient information to generate an evidence-based management strategy for idiopathic intracranial hypertension. There is inadequate information regarding which treatments are truly beneficial and which are potentially harmful. Properly designed and executed trials are needed”1 In April, 2014, IIHTT results were published in JAMA.2 This trial gave us the first evidenced-based approach relating to a protocol that significantly improved visual function in IIH.
The Idiopathic Intracranial Hypertension Treatment Trial
Quincke in 1897 reported the first cases of IIH shortly after he introduced the lumbar puncture into medicine. It was named pseudotumor cerebri in 1904 but was not well delineated clinically until the 1940's when cerebral angiography was added to pneumoencephalography to identify cases of cerebral mass lesions. Foley coined the term benign intracranial hypertension in 1955 but reports from the 1980's demonstrated a high incidence of visual loss3,4 and the term "benign" is no longer appropriate.
Lubow and Kuhr5 reported a series of IIH patients, many of whom were treated successfully with acetazolamide and weight reduction.6 The latter, another mainstay of medical therapy was further shown to be a viable treatment by others. Gücer and Viernstein7 used intracranial pressure monitoring and showed gradual CSF pressure reduction in patients receiving acetazolamide once they reached a dosage of three to four grams per day. These uncontrolled studies were the basis for using acetazolamide in the maximally tolerated dosage to treat IIH.
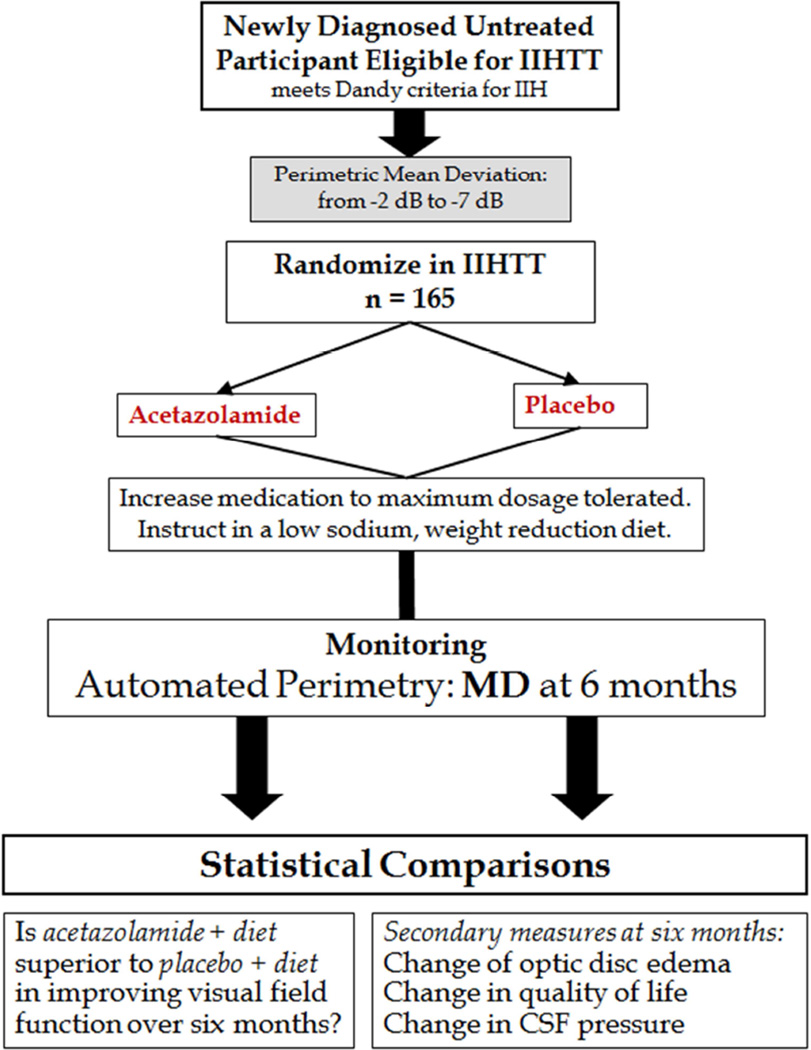
The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT), is a multicenter, double-blind, randomized, placebo-controlled study of acetazolamide in IIH subjects with mild visual loss.2,8 The trial structure and flow is found in Figure 1. All subjects received a lifestyle modification program that included weight-reduction with a low sodium diet. The purpose of the trial was to determine the effect of acetazolamide in reducing or reversing visual loss after 6 months of treatment.
Figure 1.
Clinical trial structure for the IIHTT.
Subjects needed to meet the modified Dandy criteria for IIH ad be aged 18–60. They needed to have reproducible mild visual loss (−2 to −7 dB perimetric mean deviation [PMD]). Participants needed to have bilateral papilledema, have an elevated CSF opening pressure, be untreated with regard to IIH, and have no secondary cause of increased intracranial pressure present.
Subjects were randomly assigned to receive a supervised lifestyle modification program that included a low sodium weight-reduction diet either with acetazolamide or with matching placebo. The initial dosage of study drug was 4 tablets daily in 2 divided doses, followed by dosage increases of 1 tablet every week up to a maximum dosage of 4 g/d for participants receiving acetazolamide. The dosage escalation was stopped if the participant’s papilledema grade became less than 1 in both eyes and the PMD improved to equal to or better than −1 dB in each eye, unless the presence of other symptoms such as headache or pulse synchronous tinnitus suggested that the dosage escalation continue.
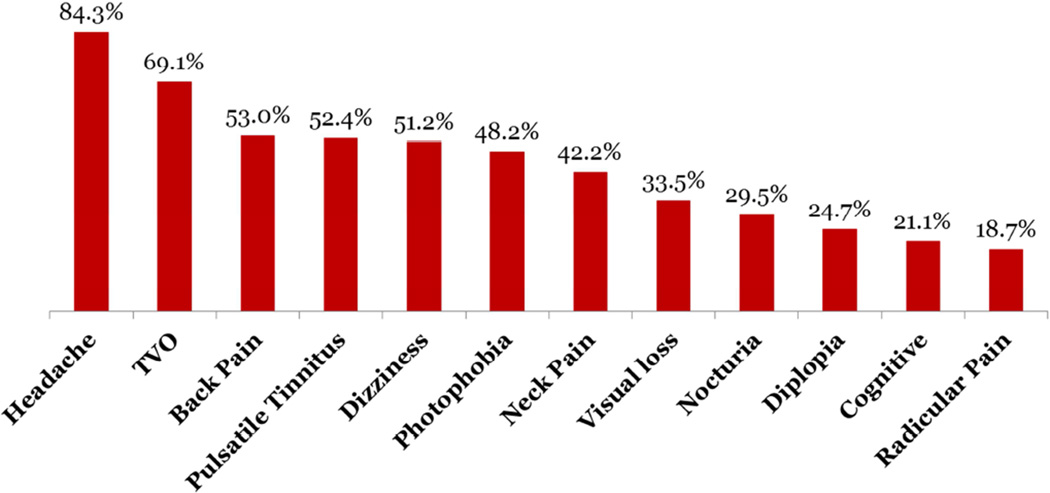
In the IIHTT, 161 women and four men were enrolled with an average age of 29.0 years (range 18–52). Figure 2 shows the frequency of symptoms at baseline. The baseline characteristics were comparable in the two treatment groups; additional baseline information is published elsewhere.9 There were 7 participants whose vision worsened to meet the endpoint of treatment failure in the trial with 6 treatment failures in the placebo group and 1 in the acetazolamide group (p = 0.06). 2,10 IIH patients with high-grade papilledema, daily transient visual obscurations and decreased visual acuity at baseline were more likely to experience treatment failure. Patients with this profile should be treated more aggressively.10
Figure 2.
Frequency of symptoms at study entry.
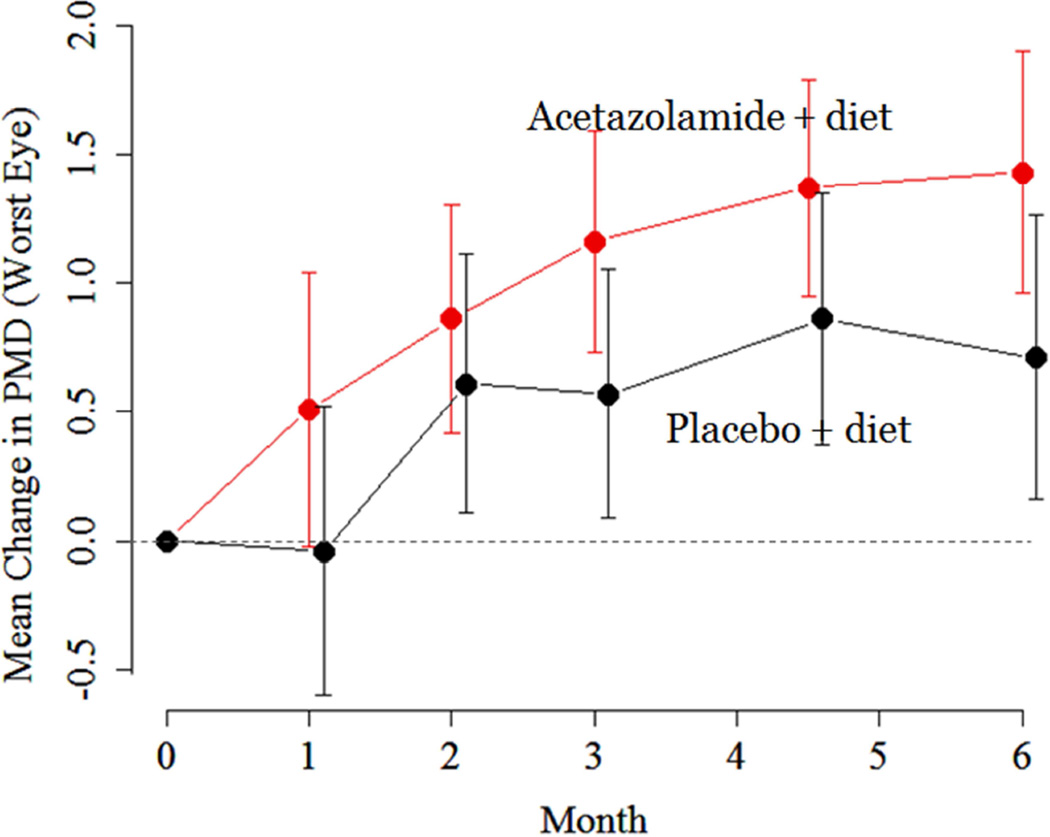
Both treatment groups experienced improvement in PMD over time in the study eye (Figure 3) with the mean improvement in the acetazolamide group being significantly larger than that in the placebo group at Month 6, p = 0.05).2 Most of the improvement related to acetazolamide took place in the first month of the intervention. Interestingly, the treatment effect on the primary outcome variable – PMD– was substantially greater (2.27 dB) in those with a baseline papilledema grade of 3–5 than in those with a baseline papilledema grade of 1–2 (−0.67 dB).2 Therefore, those with moderate to high grade papilledema are the patients that benefit most from treatment with maximally tolerated dosages of acetazolamide.
Figure 3.
Perimetric mean deviation change over six months in the IIHTT.
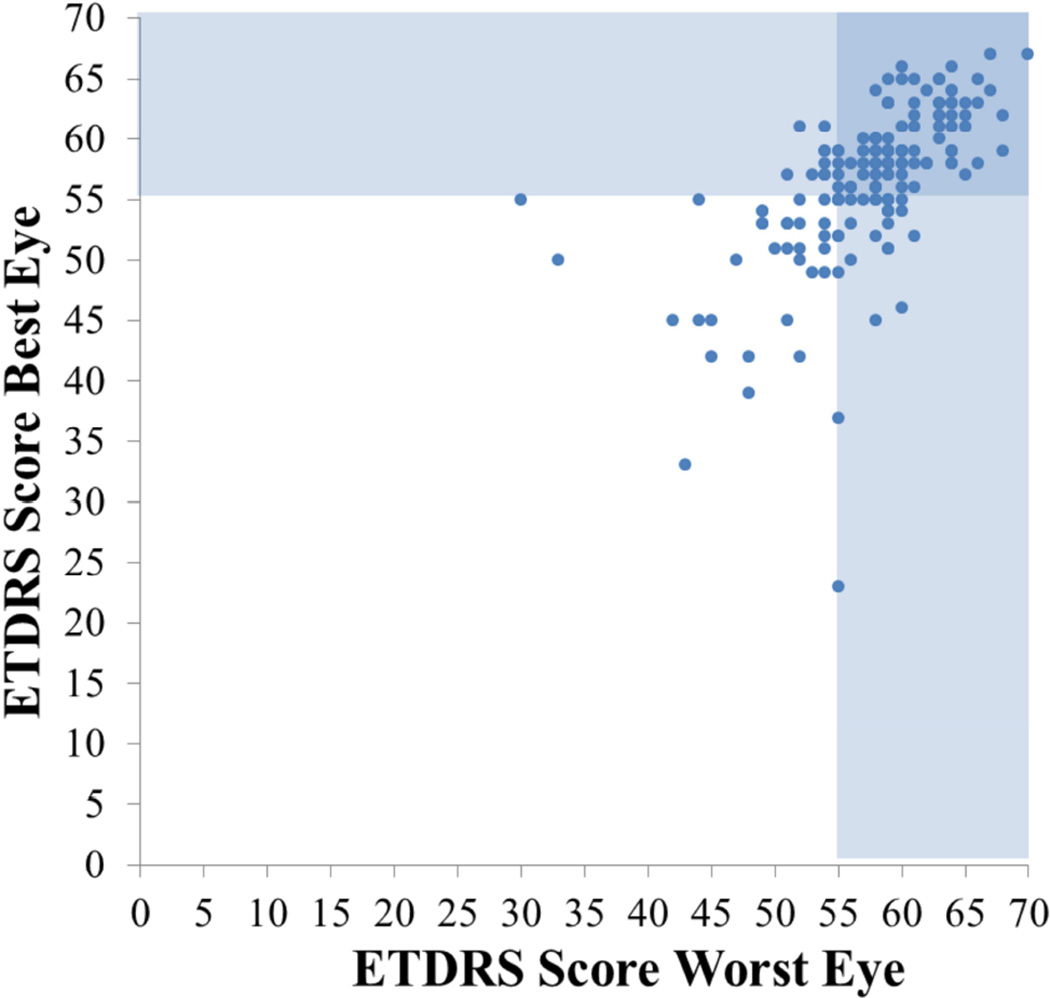
Visual acuity was mildly decreased at baseline (Figure 4). This is especially notable when one factors in that visual acuity should be at least 20/15 in this age group. With treatment acuity improved modestly but the change was not statistically significant at six months. Perimetry is a much better measure to use to follow IIH patients than visual acuity.
Figure 4.
ETDRS score of worst eye plotted against best eye. Shaded areas indicate vision of 20/20 or better.
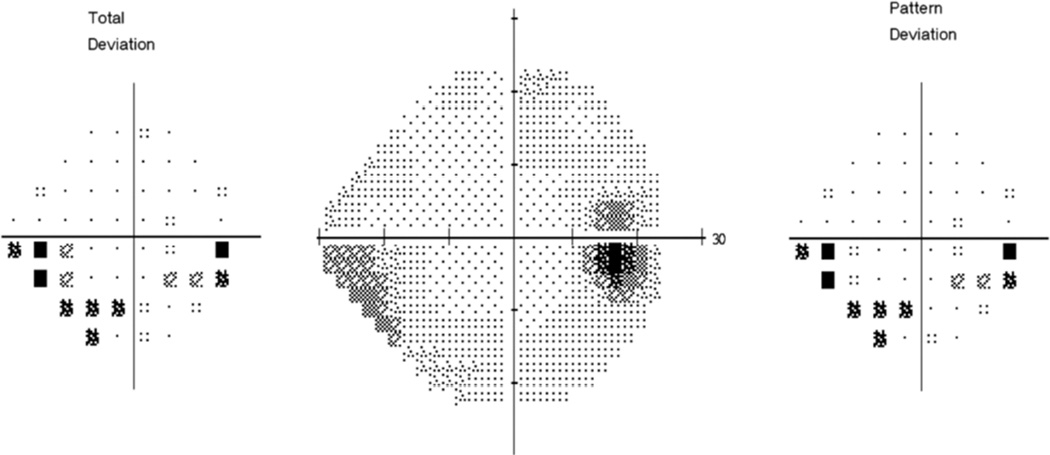
The visual field defects found at baseline were typical for IIH.11 The prototype defect was an enlarged blind spot coupled with an inferior nasal nerve fiber bundle defect (Figure 5).
Figure 5.
The prototype visual field defect in IIH patients with mild loss: the inferior nasal arcuate defect. Not the arcuate pattern of the abnormal test locations flagged on the Total Deviation plot
However, there was usually mild loss across the visual field although in the more central portions of the visual field, the loss did not reach the 95 percentile cutoff for abnormality.
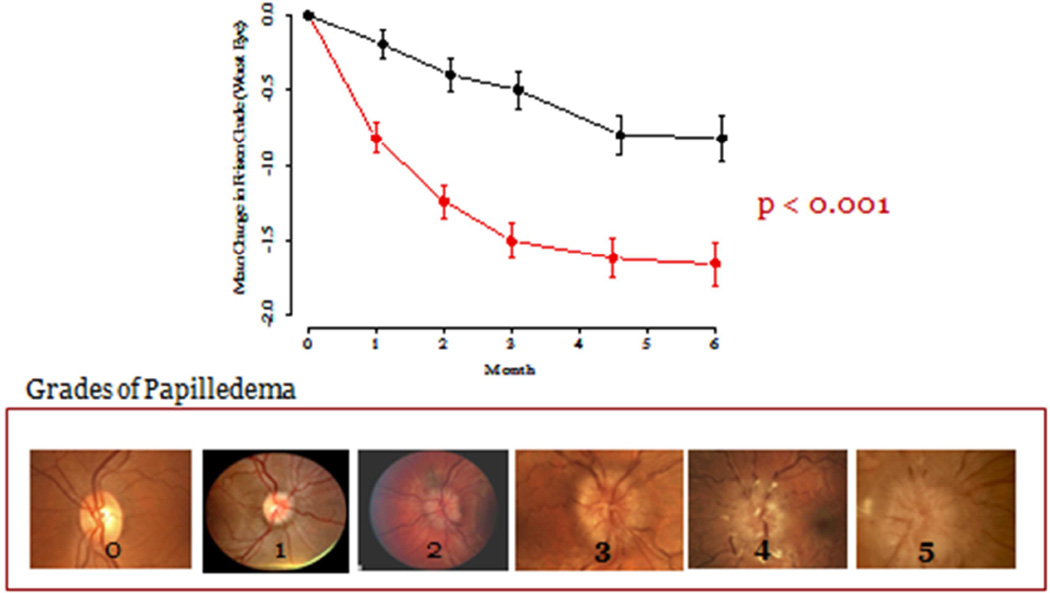
There was significant improvement in Frisén papilledema grade associated with acetazolamide treatment in the study eye and in the fellow eye (Figure 6). As with mean deviation, most of the benefit from acetazolamide was in the first month.
Figure 6.
Change in papilledema grade in the eye with the worst initial mean deviation over six months.
Acetazolamide-treated participants also experienced significant improvement in quality of life measures, including the VFQ-25 total score and its 10-item neuro-ophthalmic supplement as well as the SF-36 Physical Component Summary and Mental Component Summary scores. Positive acetazolamide-related effects on QOL appeared to be primarily mediated by improvements in visual field, neck pain, pulsatile tinnitus, and dizziness/vertigo that outweighed the side-effects of acetazolamide. No significant acetazolamide treatment effects were noted with respect to headache disability (HIT-6 total score) as both treatment groups had improved HIT scores. This may be due to subjects having concomitant analgesic rebound headaches and both groups received additional headache medications (usually naproxen sodium or low dosage amitriptyline).
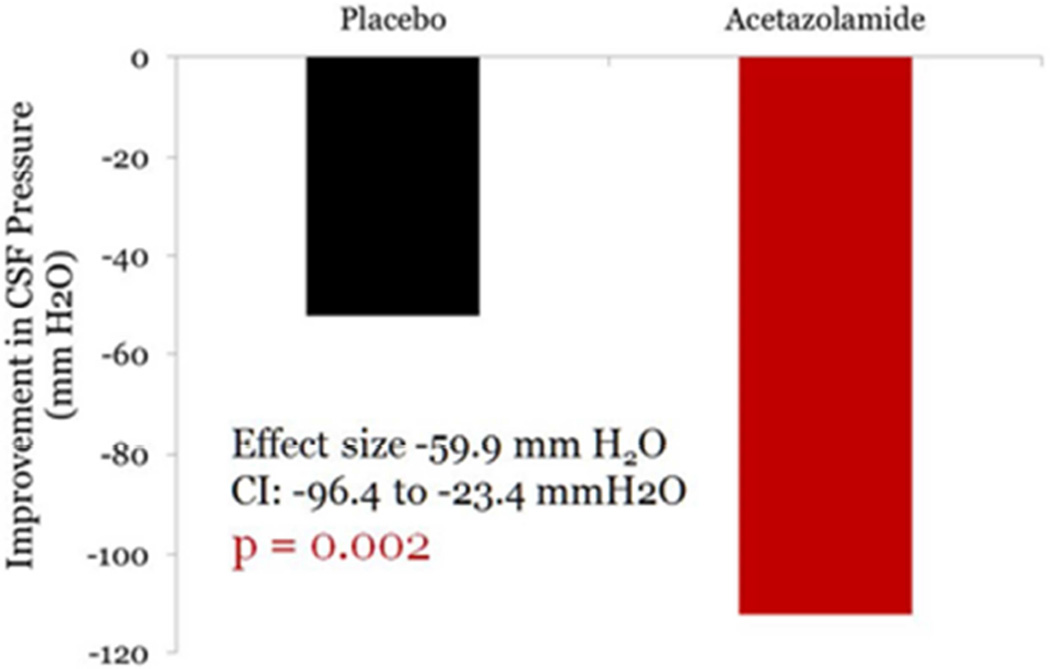
With regard to CSF pressure, the decrease in CSF pressure in the acetazolamide group was −112.3 mm H2O and was −52.4 mm H2O in the placebo group giving a treatment effect of −59.9 H2O, p = 0.002 – Figure 7).
Figure 7.
Reduction in CSF pressure over six months in the placebo and acetazolamide groups in the IIHTT.
Clinical improvement in IIH has been reported to be associated with about 6% weight loss.12 Participants on acetazolamide lost more weight over 6 months (mean −7.50 kg, from 107.72 kg to 100.22 kg) than those on placebo (mean −3.45 kg, from 107.72 kg to 104.27 kg) (treatment effect −4.05, p < 0.001. Acetazolamide also led to reductions in waist circumference and systolic and diastolic blood pressure.2 A mediation analysis concluded the effect of acetazolamide on improved visual function was independent of the amount of weight loss.
Misconceptions about Weight Loss and the IIHTT: There have been some misconceptions about what the IIHTT found with regard to weight loss. The trial did not compare acetazolamide with weight loss. It compared acetazolamide with placebo in the setting where all were receiving a weight loss intervention. The IIHTT cannot estimate an effect of weight loss as we did not design our study to determine the effect of weight loss. We did not find that both acetazolamide and weight loss improved visual field function; acetazolamide improved visual field function in a setting of weight reduction. Weight loss might have improved vision as well, but we cannot determine this without studying people who did not have a weight loss intervention.
We also did not show what percent weight loss is required to have resolution of IIH. We do not know if these people would have had resolution even without a weight loss intervention since we did not have this control group. Also, we don't know if acetazolamide would work the same way if given in a setting without a concurrent weight loss intervention.
In the trial, 48 eyes from 35 patients met visual field criteria for possible treatment failure. Seven subjects were found to have treatment failure. Upon retest, these other subjects had their PMD return to acceptable limits. Four of the variable performance subjects had large changes on retest and were reviewed by the adjudication committee and determined to be "performance failures" (temporary substantial worsening of PMD).13
Adverse events that occurred in > 5% of study participants did not cause permanent morbidity including 9 serious adverse events.14 While paresthesia, dysgeuesia, vomiting and diarrhea, nausea and fatigue were higher in the acetazolamide group than in the placebo group, quality of life measures were superior in the acetazolamide group. A mild decrease in mean potassium level was also seen with acetazolamide, but this did not require potassium supplementation in any participants. No significant changes in sodium levels or in liver function tests were apparent with acetazolamide except for the case noted above.
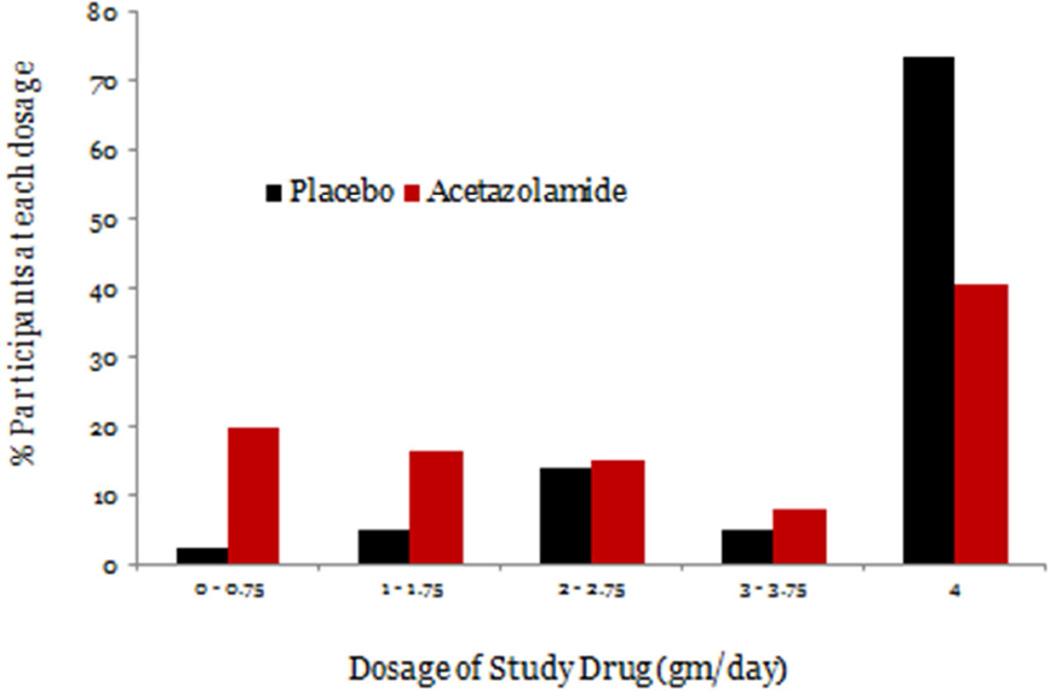
Average compliance (as measured by counts of dispensed and returned pills) was 89% in the acetazolamide group and 93% in the placebo group. The mean (SD) dosage of study medication that participants were taking at the conclusion of their participation was 2.5 g (1.5 g) in the acetazolamide group and 3.5 g (1.1 g) in the placebo group (Figure 8).
Figure 8.
Final dosage reached for placebo and acetazolamide subjects in the IIHTT.
A limitation of the study is the 19% withdrawal rate, although the frequency of and reasons for withdrawal were similar in the two treatment groups. This rate may be due, in part, to the intensity of the visit schedule. More subjects on acetazolamide than on placebo discontinued treatment, most of whom completed follow-up, which may have attenuated the estimated treatment effect.
The IIHTT showed statistically significant effects of acetazolamide to:
|
The results of the IIHTT, a multicenter randomized, double-masked, placebo-controlled study of acetazolamide in subjects with mild visual loss, demonstrate improvements in visual field function, papilledema grade, and quality of life measures. We recommend using the maximally tolerated dosage, up to 4 grams daily, of acetazolamide with a low sodium, weight reduction diet in IIH patients with mild visual loss.
What have we learned from the IIHTT:
Acetazolamide when used in IIH patients with mild visual loss produces a modest improvement in PMD over six months. The improvement is much greater in subjects with moderate to high grade papilledema.
Acetazolamide has its greatest effect on visual field function and papilledema in the first month of escalating dosage.
Acetazolamide-plus-diet patients lost twice as much weight as placebo-plus-diet patients but the acetazolamide effect on PMD was independent of the weight loss.
Treatment failure was much less common in the acetazolamide-plus-diet group compared to the placebo-plus-diet group and risk factors for treatment failure were presence of high grade papilledema and lower ETDRS visual acuity measures at baseline.
Many IIHTT subjects tolerated maximal dosages of acetazolamide. While there were many expected side effects, quality of life measures were significantly better in the acetazolamide-plus-diet group. There was no permanent morbidity from acetazolamide use.
Positive acetazolamide-related effects on quality of life appeared to be primarily mediated by improvements in visual field, neck pain, pulsatile tinnitus, and dizziness/vertigo that outweighed the side-effects of acetazolamide.
IIH patients on acetazolamide as the only diuretic do not need potassium supplementation.
Perimetry performance failures were common and were characterized by major worsening of the PMD with no change or improvement in other clinical measures.
Other recent advances in IIH
Ocular Coherence Tomography (OCT)
Optical coherence tomography (OCT) is a non-invasive high resolution (micron scale) optic nerve and retinal microstructure imaging procedure that uses light waves to take cross-section pictures by measuring backscattered or back-reflected light. There have been a variety of advances in OCT measures related to IIH over the past few years.15–17 For example, the subsurface contour of the peripapillary retinal pigment epithelium / basement membrane junction has been shown to change with CSF pressure lowering interventions.18 This can be considered a marker of structural change in IIH related to papilledema. Also, changes in the peripapillary retinal nerve fiber layer and optic nerve head volume correlate well with changes and papilledema grade. These measures provide a continuous variable rather than a categorical one and show much promise especially for use in clinical trials. Care must be taken in individual subjects though since damage to retinal ganglion cell axons will also result in decreased optic nerve head volume.15–17,19 These OCT methods are both sensitive to change and have excellent retest variability. In addition, the ganglion cell layer analysis may also show evidence of damage to the central 7° of the visual field but care must be used in its interpretation due to the presence of imaging artifacts when there is moderate to severe optic disc edema present.
Choroidal wrinkles and folds are a common accompaniment to papilledema occurring in about 40% of IIH patients. Sibony and colleagues20 have studied choroidal folds in IIHTT subjects with both fundus photos and OCT. They identified three types of folds: peripapillary wrinkles occurring in 26% of photos, retinal folds in 19% of photos and choroidal folds in 1%. While 41% of patients have wrinkles or folds with photos, 73% have them with OCT.20 The presence of these wrinkles and folds is an important feature in differentiating true optic disc edema from pseudopapilledema.
Medical Therapies
Motivational Interviewing (MI) and Weight Loss. MI is a counseling approach used to elicit behavior change that uses a collaborative, patient-centered form of guiding to elicit and strengthen motivation for change. It relies on the presence of ambivalence toward the goal (by discussing and making a choice between the pros and cons of losing weight) and assists the patient to actively develop their own plan to improve their health. A meta-analysis of RCTs using MI techniques to aid weight loss showed a reduction in body weight for intervention group compared with controls of 3.24 lbs.21
Sinclair and coworkers22 prospectively studied intracranial pressure in women with idiopathic intracranial hypertension treated with a low energy diet (a diet of foodstuffs with high volume and low calories like fresh fruits and vegetables). 25 women with BMI >25, with IIH were treated with a 425 kcal/day diet. They found with significant reductions in weight (mean 15.7 kg, intracranial pressure fell (mean 80 cm water, p < 0.001. Three months after the diet was discontinued, they showed no significant change in weight and the improvement in CSF pressure was maintained.
Surgical Therapies
Case series continue to be published regarding various surgical therapies. Fonseca et al. compared post optic nerve sheath fenestration (ONSF) visual function with post CSF shunt visual function and found a trend toward worse preoperative acuity in the ONSF cohort. Post-op mean deviation improved by 6.35 dB in the shunted group and 6.21 dB in the ONSF group. Rizzo and coworkers reported shunting results on visual field function in 15 IIH patients. The mean visual field mean deviation improvement was 5.63 dB. They conclude CSF shunting results in improvement in perimetry, RNFL swelling, and papilledema grade in patients with IIH. Huang et al.23 studied 19 shunted IIH patients and found significant improvements in acuity and but not visual field function (some of the subjects had already failed optic nerve sheath fenestration). These three papers are the first ones to investigate pre and post-op automated visual field function in CSF shunting for IIH.
It has been known for years that CSF shunting only relieved headache in about half of IIH patients.24 de Souza and colleagues studied shunted patients with the diagnosis of medication overuse headache. In 180 shunted patients, 8.3% had medication overuse headaches and 12 of the 15 patients had undergone multiple shunt revisions. They concluded shunt patients should be counselled regarding medication overuse headaches.25
There continue to be small uncontrolled case series of IIH patients treated with stenting of the lateral (transverse) venous sinus; there are many apparent successes and occasional morbidity with repeat stenting occasionally needed. Subdural hematomas, subarachnoid hemorrhage, malignant cerebral edema and prolonged anticoagulation temper the enthusiasm for this procedure. The pros and cons are discussed in a recent review.26 While a biologically plausible rationale for stenting selected IIH patients with bilateral transverse sinus stenosis, refractory to medical treatment exists, until there is a randomized controlled clinical trial evidence to document efficacy, its place in the IIH treatment armamentarium remains uncertain.
A meta-analysis of 457 articles on surgical treatment of IIH yielded 30 studies with meaningful data all with Class III evidence of efficacy. 332 patients were treated by optic nerve sheath fenestration (ONSF), 287 by lumboperitoneal shunt (LPS), 61 by ventriculoperitoneal shunt (VPS), and 88 by dural venous sinus stenting. Visual acuity improved in 49.3%, 56.6%, 67.2% and 84.6% of patients following VPS, LPS, ONSF, and stent placements, respectively in these highly selected series. Shunt revision was more frequent for LPS compared to VPS. Similar improvement in visual outcomes occurred across treatment strategies. The authors conclude there is insufficient evidence to recommend or reject any treatments modalities for IIH.27
Bariatric surgery has been used successfully to treat IIH for many years. Fridley et al. 28 reviewed the literature on the effectiveness of bariatric surgery for obese patients with idiopathic intracranial hypertension. They found 11 relevant publications reporting a total of 62 patients. The Roux-en-Y gastric bypass was the most common bariatric procedure used. Fifty-six (92%) of 61 patients with recorded postoperative clinical history had resolution of their presenting IIH symptoms following bariatric surgery. Thirty-four (97%) of 35 patients who had undergone pre- and postoperative funduscopy were found to have resolution of papilledema with the procedure. Eleven (92%) of 12 patients who had undergone pre- and postoperative formal visual field testing had complete or nearly complete resolution of visual field deficits. In 13 patients both pre- and postoperative CSF pressures were recorded, with an average postoperative pressure decrease of 254 mm water. The authors conclude published Class IV evidence suggests that bariatric surgery may be an effective treatment for IIH in morbidly obese patients, both in terms of symptom resolution and visual outcome. We discuss gastric surgery in our IIH patients with morbid obesity (BMI greater than 40).
New neuro-radiologic findings
Butros and coworkers have shown that chronically elevated CSF pressure can lead to osseous erosions including widening of the foramen ovale and other canals and ostia. This may serve as a new imaging marker for IIH. But, the foramen ovale can be difficult to visualize on standard MRI scans. The authors found average foramen ovale sizes were increased in patients with IIH compared to controls with a sensitivity of 50% and 81% specificity to detect IIH. Sensitivity and specificity of empty sella (65.9% vs. 0%), posterior globe flattening (65.9% vs. 4.5%), vertical tortuosity of the optic nerve (54.5% vs. 9.1%), and optic nerve sheath distention (52.3% vs. 11.4%, were statistically sifnificant.29
Accuracy of MRI in the diagnosis of IIH was also studied by Maralani et al.30 In this study, 43 IIH cases and 43 controls had MRI and MRV. Partially empty sella had a sensitivity of 65% to detect IIH with specificity of 95.3%; flattening of the posterior globes: 54% and 100%; combined stenosis score <4: 63% and 100% The presence of one sign, or any combination, significantly increased the odds of a diagnosis of IIH Their absence, however, did not rule out IIH.
Berdahl and colleagues.31 showed that BMI has a linear relationship with CSF pressure. They retrospectively studied 4235 patients undergoing lumbar puncture done at the Mayo Clinic that also had data to calculate a BMI. The increase in CSF pressure with increasing BMI was linear with an (p < 0.001). CSF pressure increased by 37.7% from BMI 18 (8.6 +/− 2.1 mm Hg) to BMI 39 (14.1 +/− 2.5 mm Hg). Unfortunately the r2 was only 0.20, limiting the utility of correcting CSF pressure for BMI.
As discussed above, the transverse sinus is narrowed with raised ICP. Rohr and coworkers have measured the full dural sinus tree in IIH using MRV:32 They studied 17 patients before and after treatment of IIH along with seven controls. They found stenoses of the transverse sinuses resulting in cranial venous outflow obstruction in 15/17 (88%) of IIH patients. They found the obstruction normalized in 7/15 cases (47%) after treatment of IIH. Cranial venous outflow obstruction was not detected in the control group. Segmentation of MRV revealed decreased dural sinus volumes in general in IIH patients compared to controls (p = 0.018). Sinus volumes increased significantly with normalization of CSF pressure, independent from resolution of transverse sinus stenoses (p = 0.007). They conclude there is a reduced volume of the venous sinus tree in IIH, which improves treatment of ICP.
Key Points.
Acetazolamide when used in IIH patients with mild visual loss produces a modest improvement in perimetric mean deviation (PMD) on visual field testing over six months. The improvement is much greater in subjects with moderate to high grade papilledema.
Acetazolamide has its greatest effect on visual field function and papilledema in the first month of escalating dosage.
Treatment failure was much less common in the acetazolamide-plus-diet group compared to the placebo-plus-diet group and risk factors for treatment failure were presence of high grade papilledema and lower visual acuity measures at baseline.
Positive acetazolamide-related effects on quality of life appeared to be primarily mediated by improvements in visual field, neck pain, pulsatile tinnitus, and dizziness/vertigo that outweighed the side-effects of acetazolamide.
High CSF pressure decreases the size of the entire dural sinus tree and also results in increasing size of skull foramen, canals and ostia.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement; no relevant disclosures
References
- 1.Lueck C, McIllwaine G. Interventions for Idiopathic Intracranial Hypertension. The Cochrane Library. 2009:1–10. [Google Scholar]
- 2.Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014:1641–1651. doi: 10.1001/jama.2014.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461–474. doi: 10.1001/archneur.1982.00510200003001. [DOI] [PubMed] [Google Scholar]
- 4.Wall M, Hart WM, Jr, Burde RM. Visual field defects in idiopathic intracranial hypertension (pseudotumor cerebri) Am J Ophthalmol. 1983;96:654–669. doi: 10.1016/s0002-9394(14)73425-7. [DOI] [PubMed] [Google Scholar]
- 5.Lubow M, Kuhr L. Pseudotumor cerebri: comments on practical management. In: Glaser JS, Smith JL, editors. Neuro-ophthalmology. IX. St. Louis: C.V. Mosby; 1976. pp. 199–206. [Google Scholar]
- 6.Newborg B. Pseudotumor cerebri treated by rice reduction diet. Arch Intern Med. 1974;133:802–807. [PubMed] [Google Scholar]
- 7.Gucer G, Vierenstein L. Long-term intracranial pressure recording in management of pseudotumor cerebri. J Neurosurg. 1978;49:256–263. doi: 10.3171/jns.1978.49.2.0256. [DOI] [PubMed] [Google Scholar]
- 8.Friedman DI, McDermott MP, Kieburtz K, et al. The Idiopathic Intracranial Hypertension Treatment Trial: Design Considerations and Methods. J Neuroophthalmol. 2014 doi: 10.1097/WNO.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 9.Wall M, Kupersmith MJ, Kieburtz KD, et al. The Idiopathic Intracranial Hypertension Treatment Trial: Clinical Profile at Baseline. JAMA Neurology. 2014 doi: 10.1001/jamaneurol.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wall M, Falardeau J, Fletcher WA, et al. Risk factors for poor visual outcome in patients with idiopathic intracranial hypertension. Neurology. 2015:799–805. doi: 10.1212/WNL.0000000000001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keltner JL, Johnson CA, Cello KE, et al. Baseline visual field findings in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) Invest Ophthalmol Vis Sci. 2014;55:3200–3207. doi: 10.1167/iovs.14-14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson LN, Krohel GB, Madsen RW, et al. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri) Ophthalmology. 1998;105:2313–2317. doi: 10.1016/S0161-6420(98)91234-9. [DOI] [PubMed] [Google Scholar]
- 13.Cello KE, Keltner JL, Johnson CA, et al. Factors Affecting Visual Field Outcomes in the Idiopathic Intracranial Hypertension Treatment Trial. J Neuroophthalmol. 2015 doi: 10.1097/WNO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 14.ten Hove MW, Friedman DI, Patel AD, et al. Safety and Tolerability of Acetazolamide in the Idiopathic Intracranial Hypertension Treatment Trial. J Neuroophthalmol. 2015 doi: 10.1097/WNO.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 15.Sibony P, Kupersmith MJ, Rohlf FJ. Shape analysis of the peripapillary RPE layer in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2011;52:7987–7995. doi: 10.1167/iovs.11-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibony P, Kupersmith MJ, Honkanen R, et al. Effects of lowering cerebrospinal fluid pressure on the shape of the peripapillary retina in intracranial hypertension. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.14-15298. IOVS-15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sibony P, Kupersmith MJ, Honkanen R, et al. Effects of lowering cerebrospinal fluid pressure on the shape of the peripapillary retina in intracranial hypertension. Invest Ophthalmol Vis Sci. 2014;55:8223–8231. doi: 10.1167/iovs.14-15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kupersmith MJ, Sibony P, Mandel G, et al. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillary retinal pigment epithelium layer in papilledema. Invest Ophthalmol Vis Sci. 2011;52:6558–6564. doi: 10.1167/iovs.10-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auinger P, Durbin M, Feldon S, et al. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part II: correlations and relationship to clinical features. Invest Ophthalmol Vis Sci. 2014;55:8173–8179. doi: 10.1167/iovs.14-14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibony PA, Kupersmith MJ, Feldon SE, et al. Retinal and Choroidal Folds in Papilledema. Invest Ophthalmol Vis Sci. 2015;56:5670–5680. doi: 10.1167/iovs.15-17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12:709–723. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. doi: 10.1136/bmj.c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang LC, Winter TW, Herro AM, et al. Ventriculoperitoneal shunt as a treatment of visual loss in idiopathic intracranial hypertension. J Neuroophthalmol. 2014;34:223–228. doi: 10.1097/WNO.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 24.McGirt MJ, Woodworth G, Thomas G, et al. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. Journal of Neurosurgery. 2004;101(4):627–632. doi: 10.3171/jns.2004.101.4.0627. [DOI] [PubMed] [Google Scholar]
- 25.deSouza RM, Toma A, Watkins L. Medication overuse headache - An under-diagnosed problem in shunted idiopathic intracranial hypertension patients. Br J Neurosurg. 2014;%19:1–5. 1–5. doi: 10.3109/02688697.2014.950633. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed R, Friedman DI, Halmagyi GM. Stenting of the transverse sinuses in idiopathic intracranial hypertension. J Neuroophthalmol. 2011;31:374–380. doi: 10.1097/WNO.0b013e318237eb73. [DOI] [PubMed] [Google Scholar]
- 27.Lai LT, Danesh-Meyer HV, Kaye AH. Visual outcomes and headache following interventions for idiopathic intracranial hypertension. J Clin Neurosci. 2014;21:1670–1678. doi: 10.1016/j.jocn.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Fridley J, Foroozan R, Sherman V, et al. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg. 2010 doi: 10.3171/2009.12.JNS09953. [DOI] [PubMed] [Google Scholar]
- 29.Butros SR, Goncalves LF, Thompson D, et al. Imaging features of idiopathic intracranial hypertension, including a new finding: widening of the foramen ovale. Acta Radiol. 2012:682–688. doi: 10.1258/ar.2012.110705. [DOI] [PubMed] [Google Scholar]
- 30.Maralani PJ, Hassanlou M, Torres C, et al. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin Radiol. 2012 doi: 10.1016/j.crad.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Berdahl JP, Fleischman D, Zaydlarova J, et al. Body mass index has a linear relationship with cerebrospinal fluid pressure. Invest Ophthalmol Vis Sci. 2012;53:1422–1427. doi: 10.1167/iovs.11-8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohr A, Bindeballe J, Riedel C, et al. The entire dural sinus tree is compressed in patients with idiopathic intracranial hypertension: a longitudinal, volumetric magnetic resonance imaging study. Neuroradiology. 2012;54:25–33. doi: 10.1007/s00234-011-0850-6. [DOI] [PubMed] [Google Scholar]