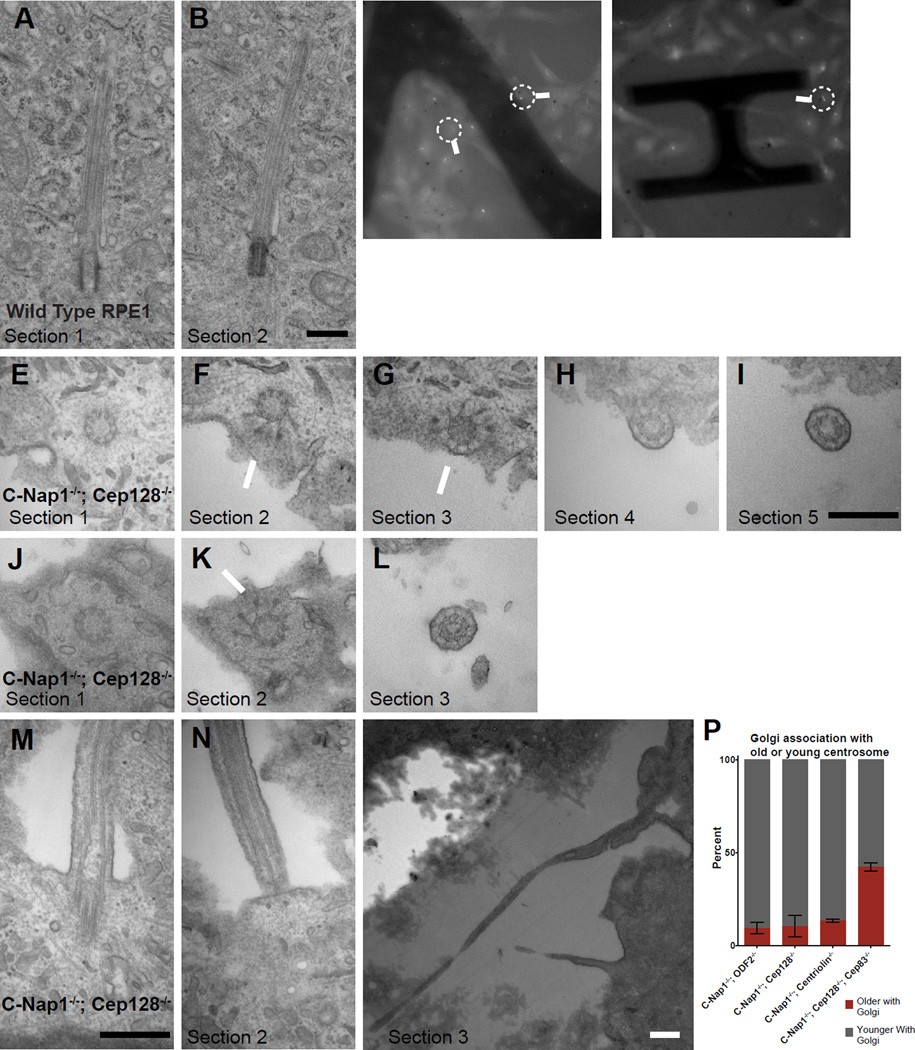
Figure 5. Loss of the deep ciliary pit in CEP128−/−; C-Nap1−/− double knockout cells.
(A and B) Two serial TEM sections of a wild type RPE1 cell carrying a submerged cilium buried in a deep membrane invagination. Scale bar represents 500nm.
(C and D) Still light microscopy images of C-Nap1−/−; CEP128−/− double knockout cells carrying either flow-sensitive or insensitive cilia, extracted from time-lapse movies (see Movie S6 and S7). Arrows mark the cells that were processed for TEM and shown in panels below as indicated.
(E, F, G and I) LM/EM analyses of a flow responsive cilium from C-Nap1−/−; CEP128−/− double mutant cells. A series of successive EM sections revealed that the cilium was at the apical surface. Section 1 contained the centriole. Section 2 and 3 showed visible distal appendages (arrows) with tip of centriole clearly on edge of the cell while the immediate next two sections showed the ciliary axoneme clearly outside of the cell. Scale bar represents 500nm.
(J, K and L) A series of EM sections of another C-Nap1−/−; CEP128−/− double mutant cilium that had been shown to respond to fluid flow under time-lapse microscopy. Sections 1 and 2 showed the distal end of the centriole while section 3 showed an axoneme outside the apical cell surface. Scale bars represent 500nm.
(M and O) Serial EM sections of a C-Nap1−/−; CEP128−/− double mutant cilium that could not respond to flow. Section 1 and 2 showed that the deep ciliary pit is disrupted, while sections 3 at a lower magnification showed that the rest of the cilium was trapped below the cell, outside of the basal cell surface. All scale bars are 500nm.
(P) The biased separation of the older/ciliated centrosome from the Golgi depends on CEP83. Quantification shows the percentage of the indicated mutant cell lines in which the older or younger centrosome associates with the Golgi. Numbers were collected from cells in which the two centrosomes are distantly separated and only one is associated with the Golgi. Older centrosomes in C-Nap1−/−; CEP128−/−; CEP83−/− triple KO cells were identified by ODF2 staining. Error bars represent standard deviations. Data of C-Nap1−/−; ODF2−/−, C-Nap1−/−; CEP128−/− and C-Nap1−/−; CNTRL−/− double KO cells are extracted from Figure 3F.