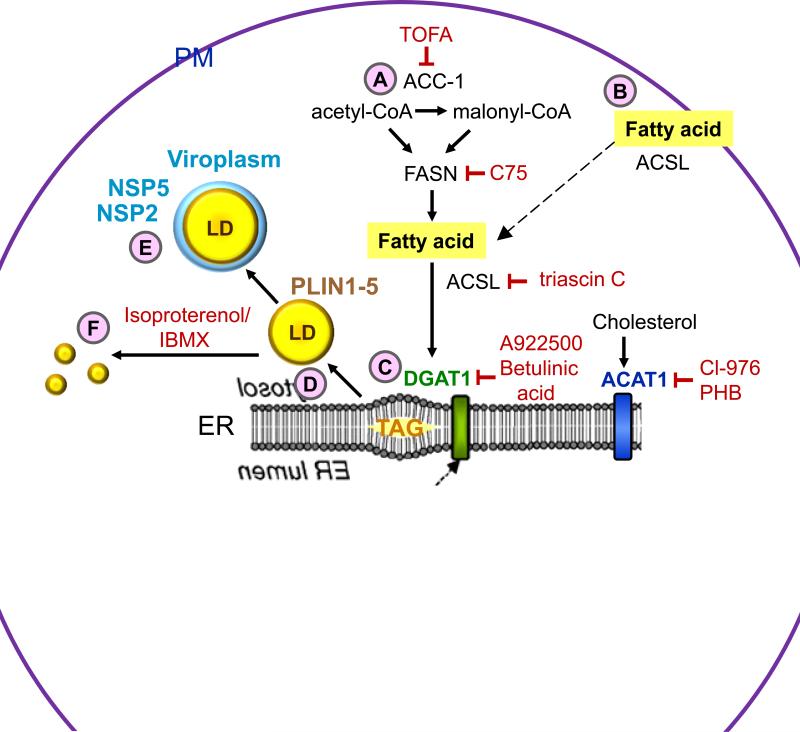
Figure 1.
Processes of lipogenesis and lipid droplet biogenesis, and interventions that disrupt viroplasm formation. (A) De novo fatty acid synthesis involves the conversion of acetyl-CoA into malonyl-CoA by acetyl-CoA carboxylase 1 (ACC-1). Fatty acid synthase (FASN) catalyzes the synthesis of the fatty acid palmitate from acetyl-CoA and malonyl-CoA. (B) Long chain acyl-CoA synthetase (ACSL) facilitates extracellular fatty acid uptake and converts fatty acids into their corresponding CoA esters for oxidation or esterification into complex lipids (e.g. triglycerides, phospholipids and cholesterol esters). (C) The ER-localized enzymes diacylglycerol acyltransferases (DGAT1 and DGAT2), and acyl-coenzyme A (CoA):cholesterol acyltransferases (ACAT1 and ACAT2) synthesize triacylglycerol (TAG) from fatty acids and sterol esters from cholesterol, respectively. These products are stored in the lipid bilayer of the ER. (D) Lipid droplets bud from the ER into the cytoplasm and acquire lipid droplet-associated proteins (PLINs 1-5). (E) Rotavirus viroplasms associate with lipid droplets. Inhibitors (shown in red) that block lipid droplet formation or disperse lipid droplets significantly decrease the number and size of viroplasms and the amount of infectious viral progeny. (F) Treatment of cells with isoproterenol and IBMX fragment lipid droplets into smaller microdroplets.